|
JAK1
JAK1 is a human tyrosine kinase protein essential for signaling for certain type I and type II cytokines. It interacts with the common gamma chain (γc) of type I cytokine receptors, to elicit signals from the IL-2 receptor family (e.g. IL-2R, IL-7R, IL-9R and IL-15R), the IL-4 receptor family (e.g. IL-4R and IL-13R), the gp130 receptor family (e.g. IL-6R, IL-11R, LIF-R, OSM-R, cardiotrophin-1 receptor (CT-1R), ciliary neurotrophic factor receptor (CNTF-R), neurotrophin-1 receptor (NNT-1R) and Leptin-R). It is also important for transducing a signal by type I (IFN-α/β) and type II (IFN-γ) interferons, and members of the IL-10 family via type II cytokine receptors. Jak1 plays a critical role in initiating responses to multiple major cytokine receptor families. Loss of Jak1 is lethal in neonatal mice, possibly due to difficulties suckling. Expression of JAK1 in cancer cells enables individual cells to contract, potentially allowing them to escape their tumor and met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oncostatin M
Oncostatin M, also known as OSM, is a protein that in humans is encoded by the ''OSM'' gene. OSM is a pleiotropic cytokine that belongs to the interleukin 6 group of cytokines. Of these cytokines it most closely resembles leukemia inhibitory factor (LIF) in both structure and function. As yet poorly defined, it is proving important in liver development, haematopoeisis, inflammation and possibly CNS development. It is also associated with bone formation and destruction. * OSM signals through cell surface receptors that contain the protein gp130. The type I receptor is composed of gp130 and LIFR, the type II receptor is composed of gp130 and OSMR. Discovery, isolation and cloning The human form of OSM was originally isolated in 1986 from the growth media of PMA treated U-937 histiocytic lymphoma cells by its ability to inhibit the growth of cell lines established from melanomas and other solid tumours. A robust protein, OSM is stable between pH2 and 11 and resistant to heating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
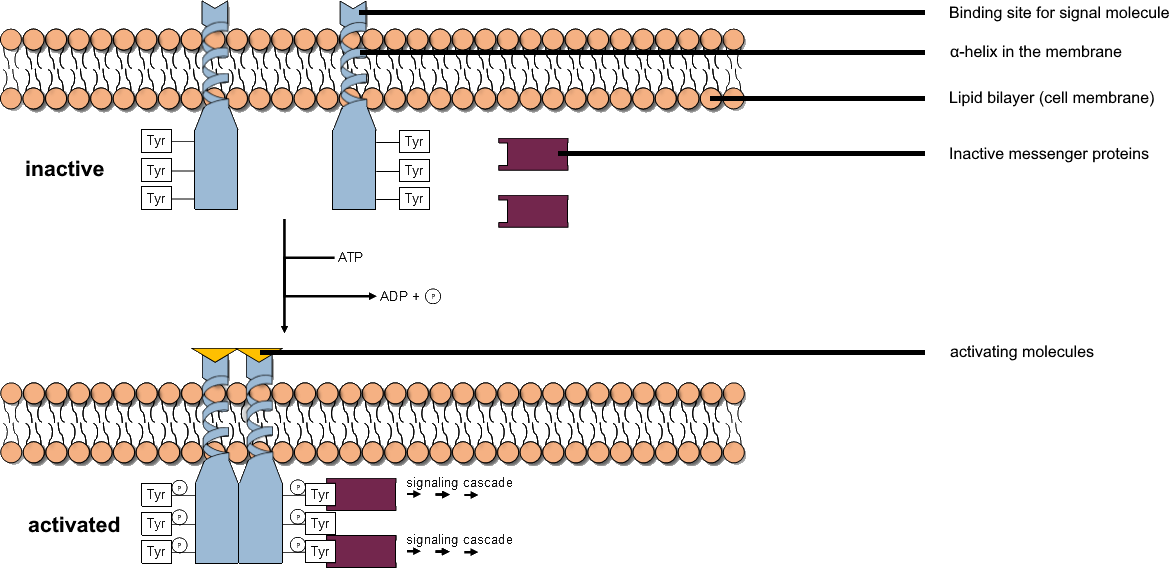
Tyrosine Kinase
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions. Tyrosine kinases belong to a larger class of enzymes known as protein kinases which also attach phosphates to other amino acids such as serine and threonine. Phosphorylation of proteins by kinases is an important mechanism for communicating signals within a cell (signal transduction) and regulating cellular activity, such as cell division. Protein kinases can become mutated, stuck in the "on" position, and cause unregulated growth of the cell, which is a necessary step for the development of cancer. Therefore, kinase inhibitors, such as imatinib and osimertinib, are often effective cancer treatments. Most tyrosine kinases have an associated protein tyrosine phosphatase, which removes the phosphate group. Reaction Protein kinases are a group of enzymes that possess a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IRS1
Insulin receptor substrate 1 (IRS-1) is a signaling adapter protein that in humans is encoded by the ''IRS-1'' gene. It is a 131 kDa protein with amino acid sequence of 1242 residues. It contains a single pleckstrin homology (PH) domain at the N-terminus and a PTB domain ca. 40 residues downstream of this, followed by a poorly conserved C-terminus tail. Together with IRS2, IRS3 (pseudogene) and IRS4, it is homologous to the ''Drosophila'' protein ''chico'', whose disruption extends the median lifespan of flies up to 48%. Similarly, Irs1 mutant mice experience moderate life extension and delayed age-related pathologies. Function Insulin receptor substrate 1 plays a key role in transmitting signals from the insulin and insulin-like growth factor-1 ( IGF-1) receptors to intracellular pathways PI3K / Akt and Erk MAP kinase pathways. Tyrosine phosphorylation of IRS-1 by insulin receptor (IR) introduces multiple binding sites for proteins bearing SH2 homology domain, such as PI3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gp130
Glycoprotein 130 (also known as gp130, IL6ST, IL6R-beta or CD130) is a transmembrane protein which is the founding member of the class of all cytokine receptors. It forms one subunit of the type I cytokine receptor within the IL-6 receptor family. It is often referred to as the common gp130 subunit, and is important for signal transduction following cytokine engagement. As with other type I cytokine receptors, gp130 possesses a WSXWS amino acid motif that ensures correct protein folding and ligand binding. It interacts with Janus kinases to elicit an intracellular signal following receptor interaction with its ligand. Structurally, gp130 is composed of five fibronectin type-III domains and one immunoglobulin-like C2-type (immunoglobulin-like) domain in its extracellular portion. Characteristics The members of the IL-6 receptor family all complex with gp130 for signal transduction. For example, IL-6 binds to the IL-6 Receptor. The complex of these two proteins then associat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoprotein 130
Glycoprotein 130 (also known as gp130, IL6ST, IL6R-beta or CD130) is a transmembrane protein which is the founding member of the class of all cytokine receptors. It forms one subunit of the type I cytokine receptor within the IL-6 receptor family. It is often referred to as the common gp130 subunit, and is important for signal transduction following cytokine engagement. As with other type I cytokine receptors, gp130 possesses a WSXWS amino acid motif that ensures correct protein folding and ligand binding. It interacts with Janus kinases to elicit an intracellular signal following receptor interaction with its ligand. Structurally, gp130 is composed of five fibronectin type-III domains and one immunoglobulin-like C2-type (immunoglobulin-like) domain in its extracellular portion. Characteristics The members of the IL-6 receptor family all complex with gp130 for signal transduction. For example, IL-6 binds to the IL-6 Receptor. The complex of these two proteins then associat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 10 Receptor, Alpha Subunit
Interleukin 10 receptor, alpha subunit is a subunit for the interleukin-10 receptor. IL10RA, is its human gene. IL10RA has also recently been designated CDW210A (cluster of differentiation W210A). Function The protein encoded by this gene is a receptor for interleukin 10. This protein is structurally related to interferon receptors. It has been shown to mediate the immunosuppressive signal of interleukin 10, and thus inhibits the synthesis of proinflammatory cytokines. This receptor is reported to promote survival of progenitor myeloid cells through the insulin receptor substrate-2/PI 3-kinase/AKT pathway. Activation of this receptor leads to tyrosine phosphorylation of JAK1 and TYK2 kinases. Interactions Interleukin 10 receptor, alpha subunit has been shown to interact with: * Interleukin 10 and * Janus kinase 1 JAK1 is a human tyrosine kinase protein essential for signaling for certain type I and type II cytokines. It interacts with the common gamma chain (γc) of type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IL-2 Receptor
The interleukin-2 receptor (IL-2R) is a heterotrimeric protein expressed on the surface of certain immune cells, such as lymphocytes, that binds and responds to a cytokine called IL-2. Composition IL-2 binds to the IL-2 receptor, which has three forms, generated by different combinations of three different proteins, often referred to as "chains": α (alpha) (also called IL-2Rα, CD25, or Tac antigen), β (beta) (also called IL-2Rβ, or CD122), and γ (gamma) (also called IL-2Rγ, γc, common gamma chain, or CD132); these subunits are also parts of receptors for other cytokines. The β and γ chains of the IL-2R are members of the type I cytokine receptor family. Structure-activity relationships of the IL-2/IL-2R interaction The three receptor chains are expressed separately and differently on various cell types and can assemble in different combinations and orders to generate low, intermediate, and high affinity IL-2 receptors. The α chain binds IL-2 with low affi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 10
Interleukin 10 (IL-10), also known as human cytokine synthesis inhibitory factor (CSIF), is an anti- inflammatory cytokine. In humans, interleukin 10 is encoded by the ''IL10'' gene. IL-10 signals through a receptor complex consisting of two IL-10 receptor-1 and two IL-10 receptor-2 proteins. Consequently, the functional receptor consists of four IL-10 receptor molecules. IL-10 binding induces STAT3 signalling via the phosphorylation of the cytoplasmic tails of IL-10 receptor 1 + IL-10 receptor 2 by JAK1 and Tyk2 respectively. Gene and protein structure The IL-10 protein is a homodimer; each of its subunits is 178-amino-acid long. IL-10 is classified as a class-2 cytokine, a set of cytokines including IL-19, IL-20, IL-22, IL-24 (Mda-7), IL-26 and interferons type-I (IFN-alpha, -beta, -epsilon, -kappa, -omega), type-II (IFN-gamma) and type-III (IFN-lambda, also known as IL-28A, IL-28B, and IL-29). Expression and synthesis In humans, IL-10 is encoded by the ''IL10'' gene, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IL2RB
Interleukin-2 receptor subunit beta is a protein that in humans is encoded by the ''IL2RB'' gene. Also known as CD122; IL15RB; P70-75. Function The interleukin 2 receptor, which is involved in T cell-mediated immune responses, is present in 3 forms with respect to ability to bind interleukin 2. The low affinity form is a monomer of the alpha subunit (also called CD25) and is not involved in signal transduction. The intermediate affinity form consists of a gamma/beta subunit heterodimer, while the high affinity form consists of an alpha/beta/gamma subunit heterotrimer. Both the intermediate and high affinity forms of the receptor are involved in receptor-mediated endocytosis and transduction of mitogenic signals from interleukin 2. The protein encoded by this gene represents the beta subunit and is a type I membrane protein. This protein also forms one of the three subunits of the IL-15 receptor. Activation of the receptor increases proliferation of CD8+ effector T cells. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interferon
Interferons (IFNs, ) are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses. IFNs belong to the large class of proteins known as cytokines, molecules used for communication between cells to trigger the protective defenses of the immune system that help eradicate pathogens. Interferons are named for their ability to "interfere" with viral replication by protecting cells from virus infections. However, virus-encoded genetic elements have the ability to antagonize the IFN response contributing to viral pathogenesis and viral diseases. IFNs also have various other functions: they activate immune cells, such as natural killer cells and macrophages, and they increase host defenses by up-regulating antigen presentation by virtue of increasing the expression of major histocompatibility complex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GNB2L1
Receptor for activated C kinase 1 (RACK1), also known as guanine nucleotide-binding protein subunit beta-2-like 1 (GNB2L1), is a 35 kDa protein that in humans is encoded by the RACK1 gene. Function RACK1 was originally isolated and identified as an intracellular protein receptor for protein kinase C, noting the significant homology to the beta subunit of heterotrimeric G proteins. Later studies established RACK1, and its yeast homolog Asc1, as a core ribosomal protein of the eukaryotic small (40S) ribosomal subunit. Much of the function of Asc1/RACK1 appears to result from its position on the 'head' of the 40S ribosomal subunit. Asc1/RACK1 participates in several aspects of eukaryotic translation and ribosome quality control, including IRES-mediated translation, non-stop decay, non-functional 18S ribosomal RNA decay, and frameshifting. Interactions RACK1 is positioned at the solvent-exposed surface of the 40S ribosomal subunit, where it is held in place through contacts ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |