|
Intramolecular Diels–Alder Cycloaddition
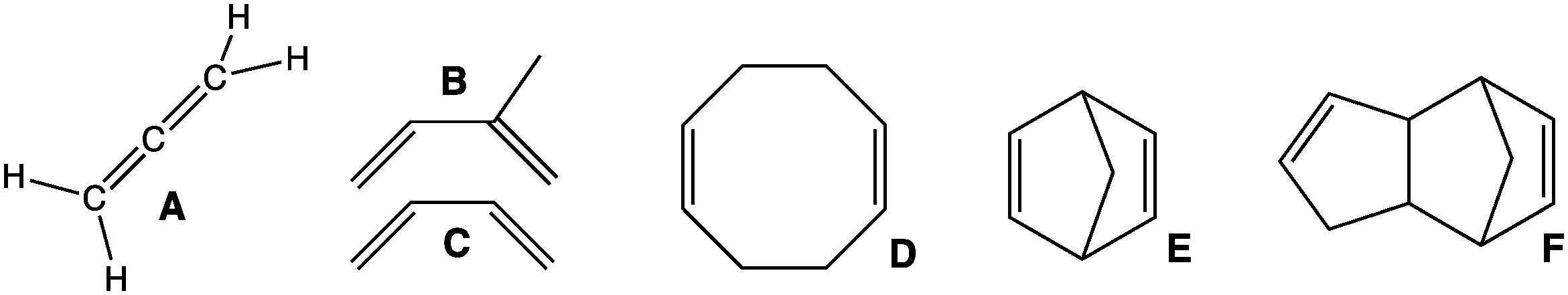
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and the dienophile are both part of the same molecule. The reaction leads to the formation of the cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system. This reaction can gives rise to various natural derivatives of decalin. Reaction products Because the two reacting groups are already attached, two basic modes of addition are possible in this reaction. Depending on whether the tether that links to the dienophile is attached to the end or the middle of the diene, fused or bridged polycyclic ring systems can be formed. The tether than attaches the two reacting groups also affects the geometry of the reaction. As a result of its Conformational isomerism, conformational and other structural restrictions, the exo vs endo results are usually not based on the simple (intermolecular) Diels–Alder rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
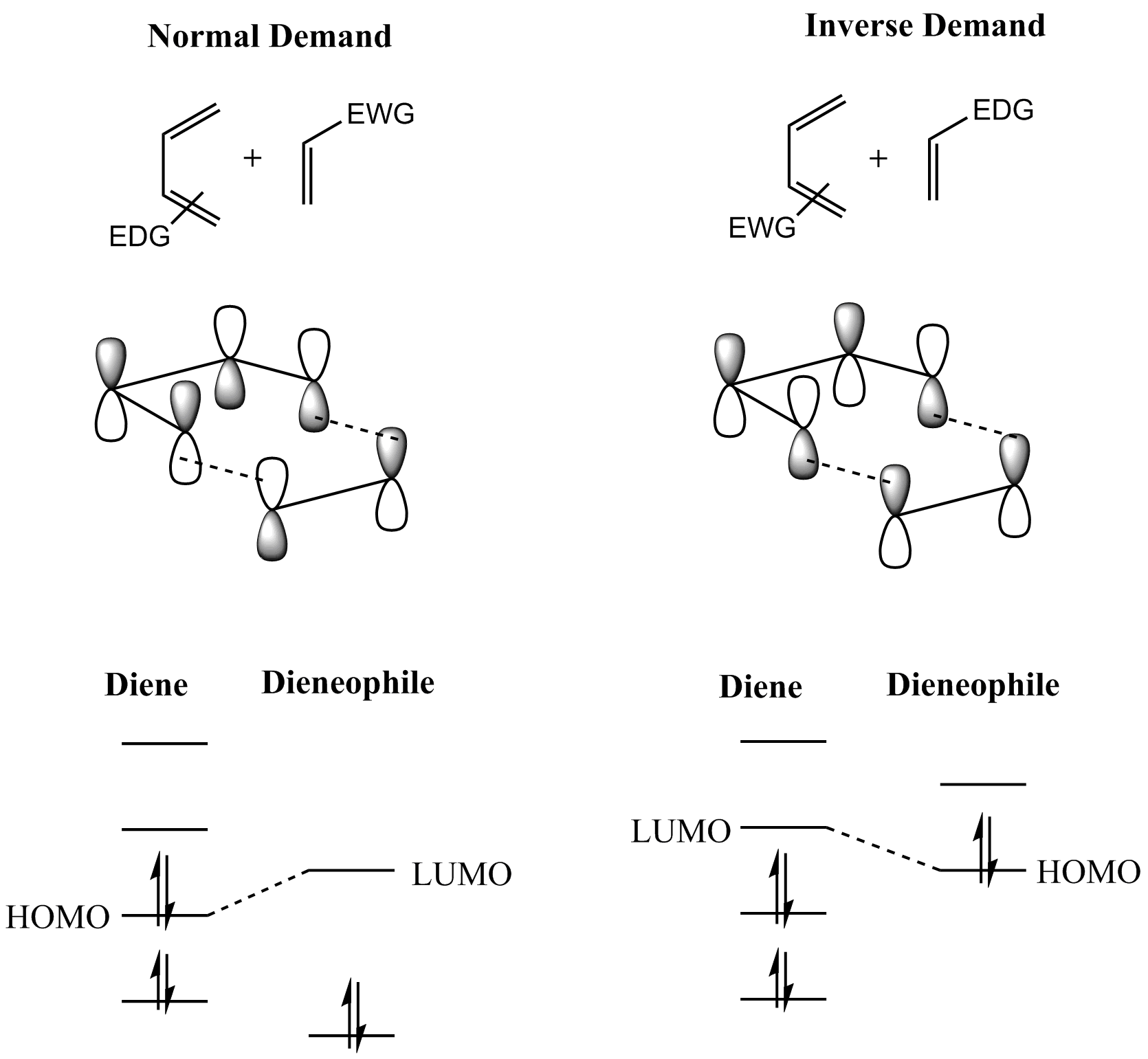
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. As a subunit of more complex molecules, dienes occur in naturally occurring and synthetic chemicals and are used in organic synthesis. Conjugated dienes are widely used as monomers in the polymer industry. Polyunsaturated fats are of interest to nutrition. Classes Dienes can be divided into three classes, depending on the relative location of the double bonds: #Cumulated dienes have the double bonds sharing a common atom. The result is more specifically called an allene. #Conjugated dienes have conjugated double bonds separated by one single bond. Conjugated dienes are more stable than other dienes because of resonance. #Unconjugated dienes have the double bonds separated by two or more single bonds. They are usually less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (molecule), water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexene
Cyclohexene is a hydrocarbon with the formula . It is a cycloalkene. At room temperature, cyclohexene is a colorless liquid with a sharp odor. Among its uses, it is an chemical intermediate, intermediate in the commercial synthesis of nylon. Production and uses Cyclohexene is produced by the partial hydrogenation of benzene, a process developed by the Asahi Chemical company. The main product of the process is cyclohexane because cyclohexene is more easily hydrogenated than benzene. In the laboratory, it can be prepared by dehydration reaction, dehydration of cyclohexanol. : Reactions and uses Benzene is converted to cyclohexylbenzene by acid-catalyzed alkylation with cyclohexene. Cyclohexylbenzene is a precursor to both phenol and cyclohexanone. Hydration of cyclohexene gives cyclohexanol, which can be dehydrogenation, dehydrogenated to give cyclohexanone, a precursor to caprolactam. The oxidative cleavage of cyclohexene gives adipic acid. Hydrogen peroxide is used as the oxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decalin
Decalin (decahydronaphthalene, also known as bicyclo .4.0ecane and sometimes decaline), a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives. Isomers Decalin occurs in ''cis'' and ''trans'' forms. The ''trans'' form is energetically more stable because of fewer steric interactions. ''cis''-Decalin is a chiral molecule without a chiral center; it has a two-fold rotational symmetry axis, but no reflective symmetry. However, the chirality is canceled through a chair-flipping process that turns the molecule into its mirror image. Image:Cis-trans isomerism of decahydronaphthalene.svg, Image:cis-decalin double chair.png, 2: Image:trans-decalin double chair.png, 3: File:Cisdecalin conformations.png, 4: ''trans''-Decalin The only possible way to join the two six-membered rings in the ''trans'' position means the second ring needs to start from two equatorial bonds (blue) of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conformational Isomerism
In chemistry, rotamers are chemical species that differ from one another primarily due to rotations about one or more single bonds. Various arrangements of atoms in a molecule that differ by rotation about single bonds can also be referred to as conformations. Conformers/rotamers differ little in their energies, so they are almost never separable in a practical sense. Rotations about single bonds are subject to small energy barriers. When the time scale for interconversion is long enough for isolation of individual rotamers (usually arbitrarily defined as a half-life of interconversion of 1000 seconds or longer), the species are termed atropisomers (''see:'' atropisomerism). The Ring flip, ring-flip of substituted cyclohexanes constitutes a common form of conformers. The study of the energetics of bond rotation is referred to as conformational analysis. In some cases, conformational analysis can be used to predict and explain product selectivity, mechanisms, and rates of reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corey 1978 Gibberellic Acid
Corey is a masculine given name and a surname. It is a masculine version of name Cora, which has Greek origins and is the maiden name of the goddess Persephone. The name also can have origins from the Gaelic word ''coire'', which means "in a cauldron" or "in a hollow". As a surname, it has a number of possible derivations, including an Old Norse personal name '' Kori'' of uncertain meaning, which is found in Scandinavia and England, often meaning curly haired. As an Irish surname it comes from Ó Comhraidhe (descendant of Comhraidheh). Notable people or fictional characters named Corey include: First name A *Corey Adam (born 1981), American stand-up comedian *Corey Adams (born 1962), Australian rugby player *Corey Adamson (born 1992), Australian baseball and Australian rules football player *Corey Allan (born 1998), Australian rugby player *Corey Allen (1934–2010), American film and television director * Corey Anderson (other), multiple people *Corey Arnold (born 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially available starting materials. Total synthesis targets can also be organometallic or inorganic. While total synthesis aims for complete construction from simple starting materials, modifying or partially synthesizing these compounds is known as semisynthesis. Natural product synthesis serves as a critical tool across various scientific fields. In organic chemistry, it tests new synthetic methods, validating and advancing innovative approaches. In medicinal chemistry, natural product synthesis is essential for creating bioactive compounds, driving progress in drug discovery and therapeutic development. Similarly, in chemical biology, it provides research tools for studying biological systems and processes. Additionally, synthesis aids natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite: both products are at least possible and merely differ in amount. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used. An enantioselective reaction is one in which one enantiomer is formed in preference to the other, in a reaction that creates an optically active product from an achiral starting material, using either a chiral catalyst, an enzyme or a chiral reagent. The degree of selectivity is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |