|
Homocysteine
Homocysteine is a non-proteinogenic α-amino acid. It is a homologue of the amino acid cysteine, differing by an additional methylene bridge (-CH2-). It is biosynthesized from methionine by the removal of its terminal Cε methyl group. In the body, homocysteine can be recycled into methionine or converted into cysteine with the aid of certain B-vitamins. High levels of homocysteine in the blood ( hyperhomocysteinemia) is regarded as a marker of cardiovascular disease, likely working through atherogenesis, which can result in ischemic injury. Therefore, hyperhomocysteinemia is a possible risk factor for coronary artery disease. Coronary artery disease occurs when an atherosclerotic plaque blocks blood flow to the coronary arteries, which supply the heart with oxygenated blood. Hyperhomocysteinemia has been correlated with the occurrence of blood clots, heart attacks, and strokes, although it is unclear whether hyperhomocysteinemia is an independent risk factor for these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperhomocysteinemia
Hyperhomocysteinemia is a medical condition characterized by an abnormally high level of homocysteine in the blood, conventionally described as above 15 μmol/L. As a consequence of the biochemical reactions in which homocysteine is involved, deficiencies of vitamin B6, folic acid (vitamin B9), and vitamin B12 can lead to high homocysteine levels. Other possible causes of hyperhomocysteinemia include genetics, excessive methionine intake, and other diseases. Hyperhomocysteinemia is typically managed with vitamin B6, vitamin B9 and vitamin B12 supplementation. Hyperhomocysteinemia is a risk factor for cardiovascular disease; however, supplements of these vitamins may not improve cardiovascular disease outcomes. Signs and symptoms Elevated levels of homocysteine have been associated with a number of disease states. Cardiovascular risks Elevated homocysteine is a known risk factor for cardiovascular disease as well as thrombosis. It has also been shown to be associate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystathionine-beta-synthase
Cystathionine-β-synthase, also known as CBS, is an enzyme () that in humans is encoded by the ''CBS'' gene. It catalyzes the first step of the transsulfuration pathway, from homocysteine to cystathionine: : L-serine + L-homocysteine \rightleftharpoons L-cystathionine + H2O CBS uses the cofactor pyridoxal-phosphate (PLP) and can be allosterically regulated by effectors such as the ubiquitous cofactor S-adenosyl-L-methionine (adoMet). This enzyme belongs to the family of lyases, to be specific, the hydro-lyases, which cleave carbon-oxygen bonds. CBS is a multidomain enzyme composed of an N-terminal enzymatic domain and two CBS domains. The ''CBS'' gene is the most common locus for mutations associated with homocystinuria. Nomenclature The List of enzymes, systematic name of this enzyme class is L-serine hydro-lyase (adding homocysteine; L-cystathionine-forming). Other names in common use include: * β-thionase, * cysteine synthase, * L-serine hydro-lyase (adding homocystei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MTHFR Metabolism
Methylenetetrahydrofolatereductase (MTHFR) is the rate-limiting enzyme in the methyl cycle, and it is encoded by the ''MTHFR'' gene. Methylenetetrahydrofolate reductase catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation to methionine. Natural variation in this gene is common in otherwise healthy people. Although some variants have been reported to influence susceptibility to occlusive vascular disease, neural tube defects, Alzheimer's disease and other forms of dementia, colon cancer, and acute leukemia, findings from small early studies have not been reproduced. Some mutations in this gene are associated with methylenetetrahydrofolate reductase deficiency. Complex I deficiency with recessive spastic paraparesis has also been linked to ''MTHFR'' variants. In addition, the aberrant promoter hypermethylation of this gene is associated with male infertility and recurrent spontaneous abortion. Bio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
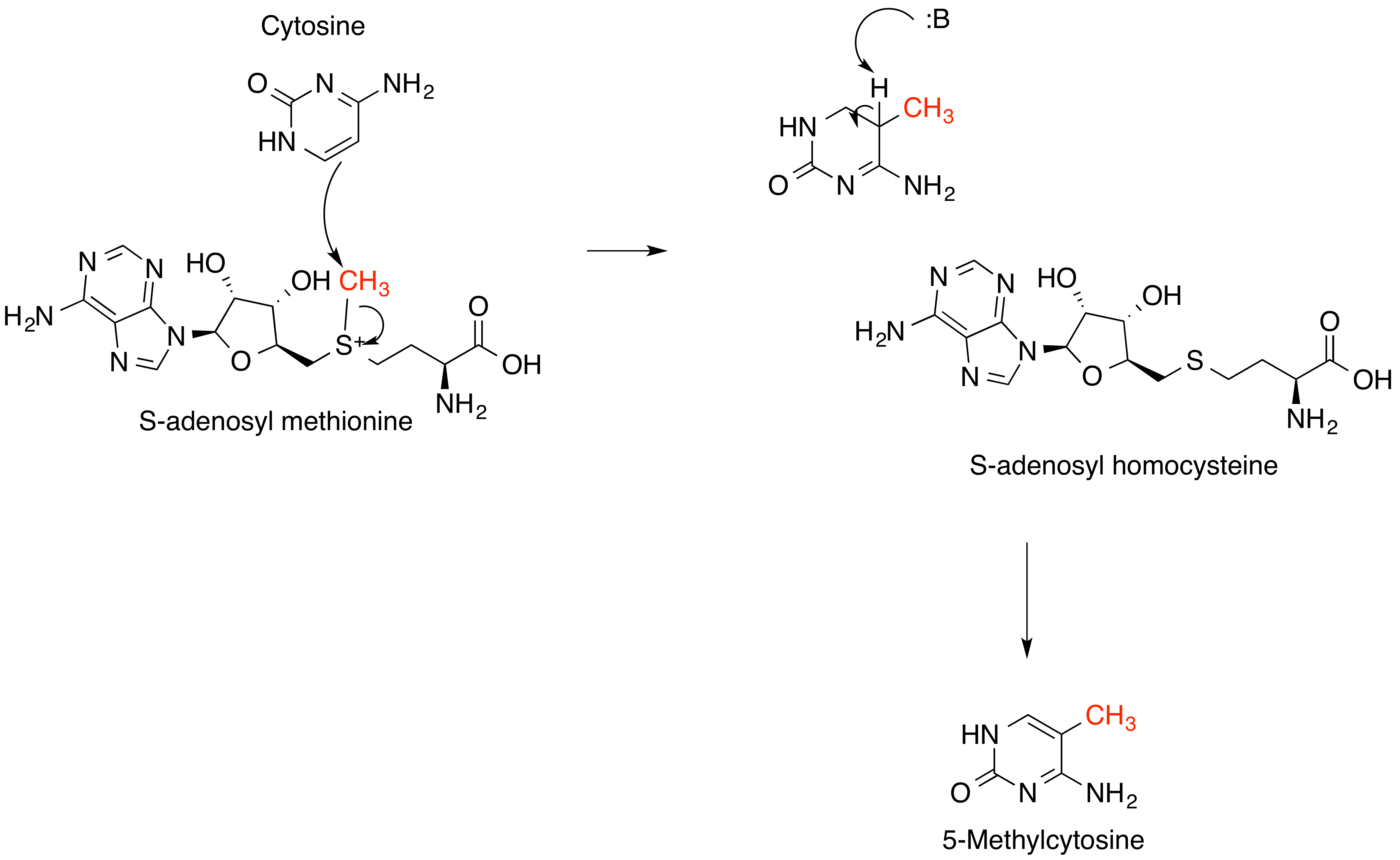
DNA Methylation
DNA methylation is a biological process by which methyl groups are added to the DNA molecule. Methylation can change the activity of a DNA segment without changing the sequence. When located in a gene promoter, DNA methylation typically acts to repress gene transcription. In mammals, DNA methylation is essential for normal development and is associated with a number of key processes including genomic imprinting, X-chromosome inactivation, repression of transposable elements, aging, and carcinogenesis. As of 2016, two nucleobases have been found on which natural, enzymatic DNA methylation takes place: adenine and cytosine. The modified bases are N6-methyladenineD. B. Dunn, J. D. Smith: ''The occurrence of 6-methylaminopurine in deoxyribonucleic acids.'' In: ''Biochem J.'' 68(4), Apr 1958, S. 627–636. PMID 13522672. ., 5-methylcytosineB. F. Vanyushin, S. G. Tkacheva, A. N. Belozersky: ''Rare bases in animal DNA.'' In: ''Nature.'' 225, 1970, S. 948–949. PMID 4391887. a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystathionine
Cystathionine is an intermediate in the synthesis of cysteine. Cystathionine is produced by the transsulfuration pathway which converts homocysteine into cystathionine. Cystathionine is then used by the enzymes cystathionine gamma-lyase (CTH), cysteine dioxygenase (CDO), and sulfinoalanine decarboxylase to produce hypotaurine and then taurine. Alternately, the cysteine from the cystathionine gamma-lyase can be used by the enzymes glutamate–cysteine ligase (GCL) and glutathione synthetase (GSS) to produce glutathione. An excess of cystathionine in the urine is called cystathioninuria. Biosynthetically, cystathionine is generated from homocysteine and serine by cystathionine beta synthase (upper reaction in the diagram below). It is then cleaved into cysteine and α-ketobutyrate by cystathionine gamma-lyase The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
B-vitamins
B vitamins are a class of water-soluble vitamins that play important roles in cell metabolism and synthesis of red blood cells. Though these vitamins share similar names (B1, B2, B3, etc.), they are chemically distinct compounds that often coexist in the same foods. In general, dietary supplements containing all eight are referred to as a vitamin B complex. Individual B vitamin supplements are referred to by the specific number or name of each vitamin, such as B1 for thiamine, B2 for riboflavin, and B3 for niacin. Some are more commonly recognized by name than by number, for example pantothenic acid, biotin, and folate. Each B vitamin is either a cofactor (generally a coenzyme) for key metabolic processes or is a precursor needed to make one and is thus an essential nutrient. List of B vitamins Note: other substances once thought to be vitamins were given numbers in the B-vitamin numbering scheme, but were subsequently discovered to be either not essential for life or m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neural Tube Defects
Neural tube defects (NTDs) are a group of birth defects in which an opening in the spine or cranium remains from early in human development. In the third week of pregnancy called gastrulation, specialized cells on the dorsal side of the embryo begin to change shape and form the neural tube. When the neural tube does not close completely, an NTD develops. Specific types include: spina bifida which affects the spine, anencephaly which results in little to no brain, encephalocele which affects the skull, and iniencephaly which results in severe neck problems. NTDs are one of the most common birth defects, affecting over 300,000 births each year worldwide. For example, spina bifida affects approximately 1,500 births annually in the United States, or about 3.5 in every 10,000 (0.035% of US births), which has decreased from around 5 per 10,000 (0.05% of US births) since folate fortification of grain products was started. The number of deaths in the US each year due to neural tub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atherogenesis
Atherosclerosis is a pattern of the disease arteriosclerosis in which the wall of the artery develops abnormalities, called lesions. These lesions may lead to narrowing due to the buildup of atheromatous plaque. At onset there are usually no symptoms, but if they develop, symptoms generally begin around middle age. When severe, it can result in coronary artery disease, stroke, peripheral artery disease, or kidney problems, depending on which arteries are affected. The exact cause is not known and is proposed to be multifactorial. Risk factors include abnormal cholesterol levels, elevated levels of inflammatory markers, high blood pressure, diabetes, smoking, obesity, family history, genetic, and an unhealthy diet. Plaque is made up of fat, cholesterol, calcium, and other substances found in the blood. The narrowing of arteries limits the flow of oxygen-rich blood to parts of the body. Diagnosis is based upon a physical exam, electrocardiogram, and exercise stress test, among ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |