|
Glyoxal
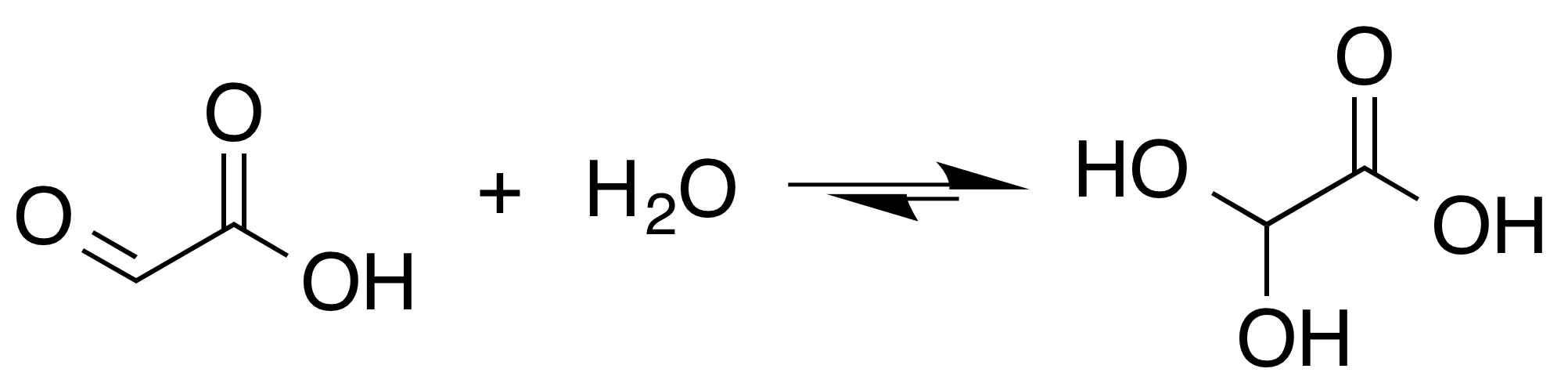
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde (a compound with two aldehyde groups). It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid is yellow, and the vapor is green.O'Neil, M.J. (2001): ''The Merck Index'', 13th Edition, page 803. Pure glyoxal is not commonly encountered because glyoxal is usually handled as a 40% aqueous solution (density near 1.24 g/mL). It forms a series of hydrates, including oligomers. For many purposes, these hydrated oligomers behave equivalently to glyoxal. Glyoxal is produced industrially as a precursor to many products. Production Glyoxal was first prepared and named by the German-British chemist Heinrich Debus (chemist), Heinrich Debus (1824–1915) by reacting ethanol with nitric acid. Commercial glyoxal is prepared either by the gas-phase oxidation of ethylene glycol in the presence of a silver or copper catalyst (the Laporte process) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylglyoxal
Methylglyoxal (MGO) is the organic compound with the formula CH3C(O)CHO. It is a reduced derivative of pyruvic acid. It is a reactive compound that is implicated in the biology of diabetes. Methylglyoxal is produced industrially by degradation of carbohydrates using overexpressed methylglyoxal synthase. Chemical structure Gaseous methylglyoxal has two carbonyl groups: an aldehyde and a ketone. In the presence of water, it exists as hydrates and oligomers. The formation of these hydrates is indicative of the high reactivity of MGO, which is relevant to its biological behavior. Biochemistry Biosynthesis and biodegradation In organisms, methylglyoxal is formed as a side-product of several metabolic pathways. Methylglyoxal mainly arises as side products of glycolysis involving glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. It is also thought to arise via the degradation of acetone and threonine. Illustrative of the myriad pathways to MGO, aristolochic acid caused 12-f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxylic Acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially. Structure and nomenclature The structure of glyoxylic acid is shown as having an aldehyde functional group. The aldehyde is only a minor component of the form most prevalent in some situations. Instead, glyoxylic acid often exists as a hydrate or a cyclic dimer (chemistry), dimer. For example, in the presence of water, the carbonyl rapidly converts to a geminal diol (described as the "monohydrate"). The equilibrium constant (''K'') is 300 for the formation of dihydroxyacetic acid at room temperature: Dihydroxyacetic acid has been characterized by X-ray crystallography. : In aqueous solution, this monohydrate exists in equilibrium with a hemiacylal dimer form:Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic compound, organic chemical compound with the chemical formula, formula , sometimes abbreviated as . It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol (drug), alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. International Agency for Research on Cancer, The International Agency for Research on Cancer (IARC) has listed acetaldehyde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylacetone
Acetylacetone is an organic compound with the chemical formula . It is classified as a 1,3-diketone. It exists in equilibrium with a tautomer . The mixture is a colorless liquid. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. Acetylacetone is a building block for the synthesis of many coordination complexes as well as heterocyclic compounds. Properties Tautomerism The Keto–enol tautomerism, keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v molecular symmetry, symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when ''K''keto→enol is equal or greater than 1, the enol form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heinrich Debus (chemist)
Heinrich Debus (13 July 1824 – 9 December 1915) was a German chemist. Education and career In 1838, he attended a trade school in Kassel, where he was taught by Robert Wilhelm Bunsen. He studied chemistry from 1845 to 1848 in Marburg, and served as Bunsen's assistant from 1847. In 1848, he earned his doctorate by investigating a red madder dye. He completed his habilitation in 1851 after Bunsen left for Breslau. At the suggestion of Frederick Augustus Genth, Debus was named Bunsen's successor at Marburg. Later in 1851 he left for England to be a chemistry teacher at Queenwood College followed from 1868 to 1870 by the position as Science Master at Clifton College, Bristol. He then taught at Guy's Hospital, London from 1870 until appointed foundation Professor of Chemistry in 1873 at the new Royal Naval College, Greenwich, where he remained until his retirement and return to Germany. In 1858, Debus first synthesized imidazole from glyoxal, ammonia, and formaldehyde. The Debu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. Silver is found in the Earth's crust in the pure, free elemental form ("native metal, native silver"), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc Refining (metallurgy), refining. Silver has long been valued as a precious metal. Silver metal is used in many bullion coins, sometimes bimetallism, alongside gold: while it is more abundant than gold, it is much less abundant as a native metal. Its purity is typically measured on a per-mille basis; a 94%-pure alloy is described as "0.940 fine". As one of the seven metals of antiquity, silver has had an enduring role in most human cultures. Other than in currency and as an in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvic Acid
Pyruvic acid (CH3COCOOH) is the simplest of the keto acids, alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate acid, conjugate base, CH3COCOO−, is an metabolic intermediate, intermediate in several metabolic pathways throughout the cell. Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or converted to fatty acids through a reaction with acetyl-CoA. It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation. Pyruvic acid supplies energy to cell (biology), cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively lactic acid fermentation, ferments to produce lactic acid, lactate when oxygen is lacking. Chemistry In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propanedial
Malondialdehyde belong to the class of β-dicarbonyls. A colorless solid, malondialdehyde is a highly reactive compound that occurs as the enol. It is a physiological metabolite, and a marker for oxidative stress. Structure and synthesis Malondialdehyde mainly exists as its enol, hydroxyacrolein:V. Nair, C. L. O'Neil, P. G. Wang "Malondialdehyde", ''Encyclopedia of Reagents for Organic Synthesis'', 2008, John Wiley & Sons, New York. Article Online Posting Date: March 14, 2008 :CH2(CHO)2 → HOC(H)=CH-CHO In organic solvents, the ''cis''-isomer is favored, whereas in water the ''trans''-isomer predominates. The equilibrium is rapid and is inconsequential for many purposes. In the laboratory it can be generated in situ by hydrolysis of its acetal 1,1,3,3-tetramethoxypropane, which is commercially available and shelf-stable, unlike malondialdehyde. Malondialdehyde is easily deprotonated to give the sodium salt of the enolate (m.p. 245 °C). Biosynthesis and reactivit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BASF
BASF SE (), an initialism of its original name , is a European Multinational corporation, multinational company and the List of largest chemical producers, largest chemical producer in the world. Its headquarters are located in Ludwigshafen, Germany. BASF comprises subsidiary, subsidiaries and joint ventures in more than 80 countries, operating six integrated production sites and 390 other production sites across Europe, Asia, Australia, the Americas and Africa. BASF has customers in over 190 countries and supplies products to a wide variety of industries. Despite its size and global presence, BASF has received relatively little public attention since it abandoned the manufacture and sale of BASF-branded consumer electronics products in the 1990s. The company began as a dye manufacturer in 1865. Fritz Haber worked with Carl Bosch, one of its employees, to invent the Haber-Bosch, Haber-Bosch process by 1912, after which the company grew rapidly. In 1925, the company merged with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trosly-Breuil
Trosly-Breuil () is a commune in the Oise department in northern France. In 1964, Canadian Jean Vanier invited two men, Raphael Simi and Philippe Seux, to leave the institutions where they lived and live with him in Trosly-Breuil. Their time together led to the establishment of L'Arche at Trosly-Breuil, a community for people with disabilities to live with those who cared for them. Since that time L'Arche communities have been established in fifty countries around the world. Population See also * Communes of the Oise department The following is a list of the 680 Communes of France, communes of the Oise Departments of France, department of France. The communes cooperate in the following Communes of France#Intercommunality, intercommunalities (as of 2025): References Communes of Oise {{Compiègne-geo-stub ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industrial Synthesis Of Glyoxal (acetaldehyde Process)
Industrial may refer to: Industry * Industrial archaeology, the study of the history of the industry * Industrial engineering, engineering dealing with the optimization of complex industrial processes or systems * Industrial city, a city dominated by one or more industries * Industrial loan company, a financial institution in the United States that lends money, and may be owned by non-financial institutions * Industrial organization, a field that builds on the theory of the firm by examining the structure and boundaries between firms and markets * Industrial Revolution, the development of industry in the 18th and 19th centuries **Second Industrial Revolution * Industrial society, a society that has undergone industrialization * Industrial technology, a broad field that includes designing, building, optimizing, managing and operating industrial equipment, and predesignated as acceptable for industrial uses, like factories * Industrial video, a video that targets “industry” as it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |