Acetaldehyde on:
[Wikipedia]
[Google]
[Amazon]
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the most important
2 CH2=CH2 + O2 -> 2 CH3CHO
In the 1970s, the world capacity of the Wacker-Hoechst direct oxidation process exceeded 2 million tonnes annually.
Smaller quantities can be prepared by the partial CH3CH2OH + 1/2 O2 -> CH3CHO + H2O
This method is one of the oldest routes for the industrial preparation of acetaldehyde.
C2H2 + Hg^2+ + H2O -> CH3CHO + Hg
The mechanism involves the intermediacy of vinyl alcohol, which
CH3CH2OH -> CH3CHO + H2
In this endothermic process, ethanol vapor is passed at 260–290 °C over a copper-based catalyst. The process was once attractive because of the value of the hydrogen coproduct,Eckert, Marc ''et al.'' (2007) "Acetaldehyde" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. but in modern times is not economically viable.
 Like many other carbonyl compounds, acetaldehyde
Like many other carbonyl compounds, acetaldehyde
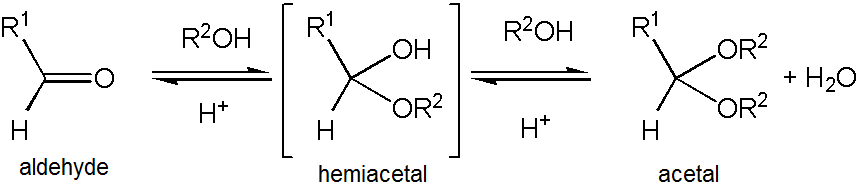 Acetaldehyde forms a stable acetal upon reaction with
Acetaldehyde forms a stable acetal upon reaction with '' )2 rather than referring to this specific compound – in fact, 1,1-diethoxyethane is also described as the diethyl acetal of acetaldehyde.
 Consumption of acetaldehyde (103 t) in 2003
Consumption of acetaldehyde (103 t) in 2003
(* Included in others -glyoxal/glyoxalic acid, crotonaldehyde, lactic acid, ''n''-butanol, 2-ethylhexanol) China is the largest consumer of acetaldehyde in the world, accounting for almost half of global consumption in 2012. Major use has been the production of acetic acid. Other uses such as
US Environmental Protection Agency In 1988 the International Agency for Research on Cancer stated, "There is ''sufficient'' evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals." p3 In October 2009 the International Agency for Research on Cancer updated the classification of acetaldehyde stating that acetaldehyde included in and generated endogenously from
 Although the levels produced by this process are minute acetaldehyde has an exceedingly low taste/ odor threshold of around 20–40 ppb and can cause an off-taste in bottled water. The level at which an average consumer could detect acetaldehyde is still considerably lower than any toxicity.
Although the levels produced by this process are minute acetaldehyde has an exceedingly low taste/ odor threshold of around 20–40 ppb and can cause an off-taste in bottled water. The level at which an average consumer could detect acetaldehyde is still considerably lower than any toxicity.
International Chemical Safety Card 0009
Methods for sampling and analysis
IARC Monograph: "Acetaldehyde"
* Hal Kibbey
Indiana University Research and Creative Activity, Vol. 17 no. 3.
* [https://web.archive.org/web/20130601111815/http://www.inclusive-science-engineering.com/acetaldehyde-production-ethylene-oxidation-stage-process/ Acetaldehyde production process flow sheet by ethylene oxidation method] {{Authority control Aldehydes Flavors Hazardous air pollutants Hepatotoxins IARC Group 2B carcinogens Organic compounds with 2 carbon atoms
aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
s, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body.
The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis (the formation of cancer). This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive subst ...
. Acetaldehyde is "one of the most frequently found air toxins with cancer risk greater than one in a million".
History
Acetaldehyde was first observed by the Swedish pharmacist/chemistCarl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydro ...
(1774); it was then investigated by the French chemists Antoine François, comte de Fourcroy and Louis Nicolas Vauquelin (1800), and the German chemists Johann Wolfgang Döbereiner (1821, 1822, 1832) and Justus von Liebig
Justus Freiherr von Liebig (12 May 1803 – 20 April 1873) was a German scientist who made major contributions to agricultural and biology, biological chemistry, and is considered one of the principal founders of organic chemistry. As a profess ...
(1835). In 1835, Liebig named it "aldehyde"; the name was later altered to "acetaldehyde".
Production
In 2003, global production was about 1 million tonnes. Before 1962,ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
and acetylene were the major sources of acetaldehyde. Since then, ethylene is the dominant feedstock
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials that are feedstock for future finished products. As feedst ...
.
The main method of production is the oxidation of ethylene
Ethylene ( IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
by the Wacker process
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst. This chemical reaction was one of ...
, which involves oxidation of ethylene using a homogeneous palladium/copper system:
:oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of ethanol in an exothermic reaction. This process typically is conducted over a silver catalyst at about 500–650 °C.
: Other methods
Hydration of acetylene
Prior to theWacker process
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst. This chemical reaction was one of ...
and the availability of cheap ethylene, acetaldehyde was produced by the hydration of acetylene. This reaction is catalyzed by mercury(II) salts:
:tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
izes to acetaldehyde. The reaction is conducted at 90–95 °C, and the acetaldehyde formed is separated from water and mercury and cooled to 25–30 °C. In the wet oxidation process, iron(III) sulfate is used to reoxidize the mercury back to the mercury(II) salt. The resulting iron(II) sulfate is oxidized in a separate reactor with nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
.
Dehydrogenation of ethanol
Traditionally, acetaldehyde was produced by the partial dehydrogenation of ethanol: :Hydroformylation of methanol
The hydroformylation of methanol with catalysts like cobalt, nickel, or iron salts also produces acetaldehyde, although this process is of no industrial importance. Similarly noncompetitive, acetaldehyde arises from synthesis gas with modest selectivity.Reactions
Tautomerization of acetaldehyde to vinyl alcohol
tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hyd ...
izes to give an enol ( vinyl alcohol; IUPAC name: ethenol):
:CH3CH=O ⇌ CH2=CHOH ∆''H''298,g = +42.7 kJ/mol
The equilibrium constant is 6 at room temperature, thus that the relative amount of the enol form in a sample of acetaldehyde is very small. At room temperature, acetaldehyde (CH3CH=O) is more stable than vinyl alcohol (CH2=CHOH) by 42.7 kJ/mol: Overall the keto-enol tautomerization occurs slowly but is catalyzed by acids.
Photo-induced keto-enol tautomerization is viable under atmospheric or stratospheric conditions. This photo-tautomerization is relevant to the earth's atmosphere, because vinyl alcohol is thought to be a precursor to carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
s in the atmosphere.
Condensation reactions
Acetaldehyde is a common electrophile inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. In condensation reactions, acetaldehyde is prochiral. It is used primarily as a source of the "CH3C+H(OH)" synthon in aldol and related condensation reactions.. Grignard reagents and organolithium compounds react with MeCHO to give hydroxyethyl derivatives. In one of the more spectacular condensation reactions, three equivalents of formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
add to MeCHO to give pentaerythritol, C(CH2OH)4.
In a Strecker reaction, acetaldehyde condenses with cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
to give, after hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis ...
, the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
alanine. Acetaldehyde can condense with amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent su ...
s to yield imines; for example, with cyclohexylamine to give ''N''-ethylidenecyclohexylamine. These imines can be used to direct subsequent reactions like an aldol condensation.
It is also a building block in the synthesis of heterocyclic compounds. In one example, it converts, upon treatment with ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
, to 5-ethyl-2-methylpyridine
5-Ethyl-2-methylpyridine is an organic compound with the formula (C2H5)(CH3)C5H3N. One of several isomeric pyridines with this formula, this derivative is of interest because it is efficiently prepared from simple reagents and it is a convenient ...
("aldehyde-collidine").
Polymeric forms
Three molecules of acetaldehyde condense to form " paraldehyde", a cyclic trimer containing C-O single bonds. Similarly condensation of four molecules of acetaldehyde give the cyclic molecule metaldehyde. Paraldehyde can be produced in good yields, using a sulfuric acid catalyst. Metaldehyde is only obtained in a few percent yield and with cooling, often using HBr rather than H2SO4 as the catalyst. At -40 °C in the presence of acid catalysts, polyacetaldehyde is produced. There are twostereomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
s of paraldehyde and four of metaldehyde.
The German chemist Valentin Hermann Weidenbusch (1821–1893) synthesized paraldehyde in 1848 by treating acetaldehyde with acid (either sulfuric or nitric acid) and cooling to 0°C. He found it quite remarkable that when paraldehyde was ''heated'' with a trace of the same acid, the reaction went the other way, recreating acetaldehyde.
Acetal derivatives
 Acetaldehyde forms a stable acetal upon reaction with
Acetaldehyde forms a stable acetal upon reaction with ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
under conditions that favor dehydration. The product, CH3CH(OCH2CH3)2, is formally named 1,1-diethoxyethane
1,1-Diethoxyethane (acetaldehyde diethyl acetal) is a major flavoring component of distilled beverages, especially malt whisky and sherry
Sherry ( es, jerez ) is a fortified wine made from white grapes that are grown near the city of Jerez de ...
but is commonly referred to as "acetal". This can cause confusion as "acetal" is more commonly used to describe compounds with the functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
s RCH(OR')2 or RR'C(ORPrecursor to vinylphosphonic acid
Acetaldehyde is a precursor to vinylphosphonic acid, which is used to make adhesives and ion conductive membranes. The synthesis sequence begins with a reaction with phosphorus trichloride: :PCl3 + CH3CHO → CH3CH(O−)PCl3+ : CH3CH(O−)PCl3+ + 2 CH3CO2H → CH3CH(Cl)PO(OH)2 + 2 CH3COCl :CH3CH(Cl)PO(OH)2 → CH2=CHPO(OH)2 + HClBiochemistry
In theliver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it ...
, the enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
alcohol dehydrogenase oxidizes ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
into acetaldehyde, which is then further oxidized into harmless acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
by acetaldehyde dehydrogenase. These two oxidation reactions are coupled with the reduction of NAD+ to NADH. In the brain, the enzyme catalase
Catalase is a common enzyme found in nearly all living organisms exposed to oxygen (such as bacteria, plants, and animals) which catalyzes the decomposition of hydrogen peroxide to water and oxygen. It is a very important enzyme in protecting t ...
is primarily responsible for oxidizing ethanol to acetaldehyde, and alcohol dehydrogenase plays a minor role. The last steps of alcoholic fermentation in bacteria, plants, and yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to consti ...
involve the conversion of pyruvate into acetaldehyde and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
by the enzyme pyruvate decarboxylase, followed by the conversion of acetaldehyde into ethanol. The latter reaction is again catalyzed by an alcohol dehydrogenase, now operating in the opposite direction.
Uses
Traditionally, acetaldehyde was mainly used as a precursor to acetic acid. This application has declined because acetic acid is produced more efficiently from methanol by theMonsanto
The Monsanto Company () was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best known product is Roundup, a glyphosate-based herbicide, developed i ...
and Cativa processes. Acetaldehyde is an important precursor to pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakl ...
derivatives, pentaerythritol, and crotonaldehyde. Urea and acetaldehyde combine to give a useful resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on n ...
. Acetic anhydride reacts with acetaldehyde to give ethylidene diacetate, a precursor to vinyl acetate, which is used to produce polyvinyl acetate
Polyvinyl acetate (PVA, PVAc, poly(ethenyl ethanoate)), commonly known as wood glue, PVA glue, white glue, carpenter's glue, school glue, or Elmer's glue in the US, is a widely available adhesive used for porous materials like wood, paper, and ...
.
The global market for acetaldehyde is declining. Demand has been impacted by changes in the production of plasticizer alcohols, which has shifted because ''n''-butyraldehyde is less often produced from acetaldehyde, instead being generated by hydroformylation of propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petrole ...
. Likewise, acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
, once produced from acetaldehyde, is made predominantly by the lower-cost methanol carbonylation process. The impact on demand has led to increase in prices and thus slowdown in the market.(* Included in others -glyoxal/glyoxalic acid, crotonaldehyde, lactic acid, ''n''-butanol, 2-ethylhexanol) China is the largest consumer of acetaldehyde in the world, accounting for almost half of global consumption in 2012. Major use has been the production of acetic acid. Other uses such as
pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakl ...
s and pentaerythritol are expected to grow faster than acetic acid, but the volumes are not large enough to offset the decline in acetic acid. As a consequence, overall acetaldehyde consumption in China may grow slightly at 1.6% per year through 2018. Western Europe is the second-largest consumer of acetaldehyde worldwide, accounting for 20% of world consumption in 2012. As with China, the Western European acetaldehyde market is expected to increase only very slightly at 1% per year during 2012–2018. However, Japan could emerge as a potential consumer for acetaldehyde in next five years due to newfound use in commercial production of butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vin ...
. The supply of butadiene has been volatile in Japan and the rest of Asia. This should provide the much needed boost to the flat market, as of 2013.
Safety
Exposure limits
The threshold limit value is 25ppm (STEL/ceiling value) and the MAK (Maximum Workplace Concentration) is 50 ppm. At 50 ppm acetaldehyde, no irritation or local tissue damage in thenasal
Nasal is an adjective referring to the nose, part of human or animal anatomy. It may also be shorthand for the following uses in combination:
* With reference to the human nose:
** Nasal administration, a method of pharmaceutical drug delivery
** ...
mucosa is observed. When taken up by the organism, acetaldehyde is metabolized rapidly in the liver to acetic acid. Only a small proportion is exhaled unchanged. After intravenous injection, the half-life in the blood is approximately 90 seconds.
Dangers
Toxicity
Many serious cases of acute intoxication have been recorded. Acetaldehyde naturally breaks down in the human body.Irritation
Acetaldehyde is an irritant of the skin, eyes, mucous membranes, throat, and respiratory tract. This occurs at concentrations as low as 1000 ppm. Symptoms of exposure to this compound includenausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. While not painful, it can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the ...
, vomiting
Vomiting (also known as emesis and throwing up) is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose.
Vomiting can be the result of ailments like food poisoning, gastroenteri ...
, and headache
Headache is the symptom of pain in the face, head, or neck. It can occur as a migraine, tension-type headache, or cluster headache. There is an increased risk of depression in those with severe headaches.
Headaches can occur as a resul ...
. These symptoms may not happen immediately. The perception
Perception () is the organization, identification, and interpretation of sensory information in order to represent and understand the presented information or environment. All perception involves signals that go through the nervous system, ...
threshold for acetaldehyde in air is in the range between 0.07 and 0.25 ppm. At such concentrations, the fruit
In botany, a fruit is the seed-bearing structure in flowering plants that is formed from the ovary after flowering.
Fruits are the means by which flowering plants (also known as angiosperms) disseminate their seeds. Edible fruits in partic ...
y odor
An odor (American English) or odour (Commonwealth English; see spelling differences) is caused by one or more volatilized chemical compounds that are generally found in low concentrations that humans and animals can perceive via their sense ...
of acetaldehyde is apparent. Conjunctiva
The conjunctiva is a thin mucous membrane that lines the inside of the eyelids and covers the sclera (the white of the eye). It is composed of non-keratinized, stratified squamous epithelium with goblet cells, stratified columnar epitheli ...
l irritations have been observed after a 15-minute exposure to concentrations of 25 and 50 ppm, but transient conjunctivitis and irritation of the respiratory tract have been reported after exposure to 200 ppm acetaldehyde for 15 minutes.
Carcinogenicity
Acetaldehyde is carcinogenic in humans.Chemical Summary For AcetaldehydeUS Environmental Protection Agency In 1988 the International Agency for Research on Cancer stated, "There is ''sufficient'' evidence for the carcinogenicity of acetaldehyde (the major metabolite of ethanol) in experimental animals." p3 In October 2009 the International Agency for Research on Cancer updated the classification of acetaldehyde stating that acetaldehyde included in and generated endogenously from
alcoholic beverage
An alcoholic beverage (also called an alcoholic drink, adult beverage, or a drink) is a drink that contains ethanol, a type of alcohol that acts as a drug and is produced by fermentation of grains, fruits, or other sources of sugar. The c ...
s is a Group I human carcinogen. In addition, acetaldehyde is damaging to DNA and causes abnormal muscle development as it binds to proteins.
DNA crosslinks
Acetaldehyde induces DNA interstrand crosslinks, a form of DNA damage. These can be repaired by either of two replication-coupled DNA repair pathways.Hodskinson MR, Bolner A, Sato K, Kamimae-Lanning AN, Rooijers K, Witte M, Mahesh M, Silhan J, Petek M, Williams DM, Kind J, Chin JW, Patel KJ, Knipscheer P. Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms. Nature. 2020 Mar;579(7800):603-608. doi: 10.1038/s41586-020-2059-5. Epub 2020 Mar 4. PMID 32132710; PMCID: PMC7116288. The first is referred to as the FA pathway, because it employs gene products defective in Fanconi's anemia patients. This repair pathway results in increased mutation frequency and altered mutational spectrum. The second repair pathway requires replication fork convergence, breakage of the acetaldehyde crosslink, translesion synthesis by a Y-family DNA polymerase and homologous recombination.Aggravating factors
Alzheimer's disease
People with a genetic deficiency for the enzyme responsible for the conversion of acetaldehyde intoacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
may have a greater risk of Alzheimer's disease. "These results indicate that the ALDH2 deficiency is a risk factor for LOAD ate-onset Alzheimer's disease..."
Genetic conditions
A study of 818 heavy drinkers found that those exposed to more acetaldehyde than normal through a genetic variant of the gene encoding for alcohol dehydrogenase are at greater risk of developing cancers of theupper gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organs of the digestive system, in humans and ...
and liver.
Disulfiram
The drug disulfiram (Antabuse) inhibits acetaldehyde dehydrogenase, an enzyme that oxidizes the compound into acetic acid. Metabolism of ethanol forms acetaldehyde before acetaldehyde dehydrogenase forms acetic acid, but with the enzyme inhibited, acetaldehyde accumulates. If one consumes ethanol while taking disulfiram, the hangover effect of ethanol is felt more rapidly and intensely. As such, disulfiram is sometimes used as a deterrent for alcoholics wishing to stay sober.Sources of exposure
Indoor air
Acetaldehyde is a potential contaminant in workplace, indoors, and ambient environments. Moreover, the majority of humans spend more than 90% of their time in indoor environments, increasing any exposure and the risk to human health. In a study inFrance
France (), officially the French Republic ( ), is a country primarily located in Western Europe. It also comprises of overseas regions and territories in the Americas and the Atlantic, Pacific and Indian Oceans. Its metropolitan ar ...
, the mean indoor concentration of acetaldehydes measured in 16 homes was approximately seven times higher than the outside acetaldehyde concentration. The living room had a mean of 18.1±17.5 μg m−3 and the bedroom was 18.2±16.9 μg m−3, whereas the outdoor air had a mean concentration of 2.3±2.6 μg m−3.
It has been concluded that volatile organic compounds (VOC) such as benzene, formaldehyde, acetaldehyde, toluene, and xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are sub ...
s have to be considered priority pollutant
A pollutant or novel entity is a substance or energy introduced into the environment that has undesired effects, or adversely affects the usefulness of a resource. These can be both naturally forming (i.e. minerals or extracted compounds like o ...
s with respect to their health effects. It has been pointed that in renovated or completely new buildings, the VOCs concentration levels are often several orders of magnitude higher. The main sources of acetaldehydes in homes include building materials, laminate, PVC flooring, varnished wood flooring, and varnished cork/pine flooring (found in the varnish, not the wood). It is also found in plastics, oil-based and water-based paints, in composite wood ceilings, particle-board, plywood, treated pine wood, and laminated chipboard furniture.
Outdoor air
The use of acetaldehyde is widespread in different industries, and it may be released into waste water or the air during production, use, transportation and storage. Sources of acetaldehyde include fuel combustion emissions from stationary internal combustion engines and power plants that burn fossil fuels, wood, or trash, oil and gas extraction, refineries, cement kilns, lumber and wood mills and paper mills. Acetaldehyde is also present in automobile anddiesel exhaust
Diesel exhaust is the gaseous exhaust produced by a diesel type of internal combustion engine, plus any contained particulates. Its composition may vary with the fuel type or rate of consumption, or speed of engine operation (e.g., idling or at ...
. As a result, acetaldehyde is "one of the most frequently found air toxics with cancer risk greater than one in a million".
Tobacco smoke
Natural tobaccopolysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with w ...
, including cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall ...
, have been shown to be the primary precursors making acetaldehyde a significant constituent of tobacco smoke. It has been demonstrated to have a synergistic effect with nicotine in rodent studies of addiction. Acetaldehyde is also the most abundant carcinogen in tobacco smoke; it is dissolved into the saliva while smoking.
Cannabis smoke
Acetaldehyde has been found in cannabis smoke. This finding emerged through the use of new chemical techniques that demonstrated the acetaldehyde present was causing DNA damage in laboratory settings.Alcohol consumption
Many microbes produce acetaldehyde from ethanol, but they have a lower capacity to eliminate the acetaldehyde, which can lead to the accumulation of acetaldehyde in saliva, stomach acid, and intestinal contents. Fermented food and many alcoholic beverages can also contain significant amounts of acetaldehyde. Acetaldehyde, derived from mucosal or microbial oxidation of ethanol, tobacco smoke, and diet, appears to act as a cumulative carcinogen in the upper digestive tract of humans. According to European Commission's Scientific Committee on Consumer Safety's (SCCS) "Opinion on Acetaldehyde" (2012) thecosmetic
Cosmetic may refer to:
* Cosmetics, or make-up, substances to enhance the beauty of the human body, apart from simple cleaning
*Cosmetic, an adjective describing beauty, aesthetics, or appearance, especially concerning the human body
*Cosmetic, ...
products special risk limit is 5 mg/L and acetaldehyde should not be used in mouth-washing products.
Plastics
Acetaldehyde can be produced by the photo-oxidation of polyethylene terephthalate (PETE), via a Type II Norrish reaction. Although the levels produced by this process are minute acetaldehyde has an exceedingly low taste/ odor threshold of around 20–40 ppb and can cause an off-taste in bottled water. The level at which an average consumer could detect acetaldehyde is still considerably lower than any toxicity.
Although the levels produced by this process are minute acetaldehyde has an exceedingly low taste/ odor threshold of around 20–40 ppb and can cause an off-taste in bottled water. The level at which an average consumer could detect acetaldehyde is still considerably lower than any toxicity.
Candida Overgrowth
''Candida albicans'' in patients with potentially carcinogenic oral diseases has been shown to produce acetaldehyde in quantities sufficient to cause problems.See also
* Alcohol dehydrogenase * Disulfiram-like drug *Formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
* Paraldehyde
* Wine fault
References
External links
International Chemical Safety Card 0009
Methods for sampling and analysis
IARC Monograph: "Acetaldehyde"
* Hal Kibbey
Indiana University Research and Creative Activity, Vol. 17 no. 3.
* [https://web.archive.org/web/20130601111815/http://www.inclusive-science-engineering.com/acetaldehyde-production-ethylene-oxidation-stage-process/ Acetaldehyde production process flow sheet by ethylene oxidation method] {{Authority control Aldehydes Flavors Hazardous air pollutants Hepatotoxins IARC Group 2B carcinogens Organic compounds with 2 carbon atoms