|
Dolomitization
Dolomitization is a geological process where magnesium ions replace calcium ions in the mineral calcite, resulting in the formation of dolomite. Dolomitization conditions are present in Abu Dhabi, the Mediterranean Sea, and some Brazilian hypersaline lagoons (most notably Lagoa Vermelha Lagoon). The areas where dolomitization take place are limited, as modern seawater is less suited to dolomite formation. This is evident in the noticeable decrease in modern dolomite depositions compared to older depositions. Dolomitization involves substantial recrystallization which can be described by the following equation: 2 CaCO3(calcite) + Mg2+ ↔ CaMg(CO3)2(dolomite) + Ca2+ The conditions for dolomitization depend on several factors, including temperature, saturation state, Mg:Ca ratio, and the presence of inhibitors and microorganisms.Microorganisms allow the precipitation of preliminary dolomite stages through certain metabolic pathways. Dolomitization occurs in kinetic intermed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dolomite (mineral)
Dolomite () is an anhydrous carbonate mineral composed of calcium magnesium carbonate, ideally The term is also used for a sedimentary carbonate rock composed mostly of the mineral dolomite (see Dolomite (rock)). An alternative name sometimes used for the dolomitic rock type is dolostone. History As stated by Nicolas-Théodore de Saussure the mineral dolomite was probably first described by Carl Linnaeus in 1768. In 1791, it was described as a rock by the French natural history, naturalist and geologist Déodat Gratet de Dolomieu (1750–1801), first in buildings of the old city of Rome, and later as samples collected in the County_of_Tyrol, Tyrolean Alps. Nicolas-Théodore de Saussure first named the mineral (after Dolomieu) in March 1792. Properties The mineral dolomite crystallizes in the trigonal, trigonal-rhombohedral system. It forms white, tan, gray, or pink crystals. Dolomite is a double carbonate, having an alternating structural arrangement of calcium and magnesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid substance with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The Geology, geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock (geology), rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral or may be an aggregate (geology), aggregate of two or more different types of minerals, spaci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microbial Mats, Laguna Negra
A microorganism, or microbe, is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from antiquity, with an early attestation in Jain literature authored in 6th-century BC India. The scientific study of microorganisms began with their observation under the microscope in the 1670s by Anton van Leeuwenhoek. In the 1850s, Louis Pasteur found that microorganisms caused food spoilage, debunking the theory of spontaneous generation. In the 1880s, Robert Koch discovered that microorganisms caused the diseases tuberculosis, cholera, diphtheria, and anthrax. Microorganisms are extremely diverse, representing most unicellular organisms in all three domains of life: two of the three domains, Archaea and Bacteria, only contain microorganisms. The third domain, Eukaryota, includes all multicellular organisms as well as many unicellular protists and protozoans that are microbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supersaturation
In physical chemistry, supersaturation occurs with a solution (chemistry), solution when the concentration of a solute exceeds the concentration specified by the value of solubility at Solubility equilibrium, equilibrium. Most commonly the term is applied to a solution of a solid in a liquid, but it can also be applied to liquids and gases dissolved in a liquid. A supersaturated solution is in a metastable state; it may return to equilibrium by separation process, separation of the excess of solute from the solution, by dilution of the solution by adding solvent, or by increasing the solubility of the solute in the solvent. History Early studies of the phenomenon were conducted with sodium sulfate, also known as Glauber's Salt because, unusually, the solubility of this salt in water may decrease with increasing temperature. Early studies have been summarised by Tomlinson. It was shown that the crystallization of a supersaturated solution does not simply come from its agitation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanogen
Methanogens are anaerobic archaea that produce methane as a byproduct of their energy metabolism, i.e., catabolism. Methane production, or methanogenesis, is the only biochemical pathway for Adenosine triphosphate, ATP generation in methanogens. All known methanogens belong exclusively to the Domain (biology), domain Archaea, although some bacteria, plants, and animal cells are also known to produce methane. However, the biochemical pathway for methane production in these organisms differs from that in methanogens and does not contribute to ATP formation. Methanogens belong to various Phylum, phyla within the domain Archaea. Previous studies placed all known methanogens into the superphylum Euryarchaeota. However, recent phylogenomic data have led to their reclassification into several different phyla. Methanogens are common in various anoxic environments, such as marine and freshwater sediments, wetlands, the Gastrointestinal tract, digestive tracts of animals, wastewater treatment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Carbon Fixation
Biological carbon fixation, or сarbon assimilation, is the process by which living organisms convert inorganic carbon (particularly carbon dioxide, ) to organic compounds. These organic compounds are then used to store energy and as structures for other biomolecules. Carbon is primarily fixed through photosynthesis, but some organisms use chemosynthesis in the absence of sunlight. Chemosynthesis is carbon fixation driven by chemical energy rather than from sunlight. The process of biological carbon fixation plays a crucial role in the global carbon cycle, as it serves as the primary mechanism for removing from the atmosphere and incorporating it into living biomass. The primary production of organic compounds allows carbon to enter the biosphere. Carbon is considered essential for life as a base element for building organic compounds. The flow of carbon from the Earth's atmosphere, oceans and lithosphere into lifeforms and then back into the air, water and soil is one of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaerobic Oxidation Of Methane
Anaerobic oxidation of methane (AOM) is a methane-consuming microbial process occurring in anoxic marine and freshwater sediments. AOM is known to occur among mesophiles, but also in psychrophiles, thermophiles, halophiles, acidophiles, and alkophiles. During AOM, methane is oxidized with different terminal electron acceptors such as sulfate, nitrate, nitrite and metals, either alone or in syntrophy with a partner organism. Coupled to sulfate reduction The overall reaction is: :CH4 + SO42− → HCO3− + HS− + H2O Sulfate-driven AOM is mediated by a syntrophic consortium of methanotrophic archaea and sulfate-reducing bacteria. They often form small aggregates or sometimes voluminous mats. The archaeal partner is abbreviated ANME, which stands for "anaerobic methanotroph". ANME's are very closely related to methanogenic archaea and recent investigations suggest that AOM is an enzymatic reversal of methanogenesis. It is still poorly understood how the syntrophic partners ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanogenesis
Methanogenesis or biomethanation is the formation of methane coupled to energy conservation by microbes known as methanogens. It is the fourth and final stage of anaerobic digestion. Organisms capable of producing methane for energy conservation have been identified only from the domain Archaea, a group phylogenetically distinct from both eukaryotes and bacteria, although many live in close association with anaerobic bacteria. The production of methane is an important and widespread form of microbial metabolism. In anoxic environments, it is the final step in the decomposition of biomass. Methanogenesis is responsible for significant amounts of natural gas accumulations, the remainder being thermogenic. Biochemistry Methanogenesis in microbes is a form of anaerobic respiration. Methanogens do not use oxygen to respire; in fact, oxygen inhibits the growth of methanogens. The terminal electron acceptor in methanogenesis is not oxygen, but carbon. The two best described pathways ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemotroph
A chemotroph is an organism that obtains energy by the oxidation of electron donors in their environments. These molecules can be organic ( chemoorganotrophs) or inorganic ( chemolithotrophs). The chemotroph designation is in contrast to phototrophs, which use photons. Chemotrophs can be either autotrophic or heterotrophic. Chemotrophs can be found in areas where electron donors are present in high concentration, for instance around hydrothermal vents. Chemoautotroph Chemoautotrophs are autotrophic organisms that can rely on chemosynthesis, i.e. deriving biological energy from chemical reactions of environmental inorganic substrates and synthesizing all necessary organic compounds from carbon dioxide. Chemoautotrophs can use inorganic energy sources such as hydrogen sulfide, elemental sulfur, ferrous iron, molecular hydrogen, and ammonia or organic sources to produce energy. Most chemoautotrophs are prokaryotic extremophiles, bacteria, or archaea that live in o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
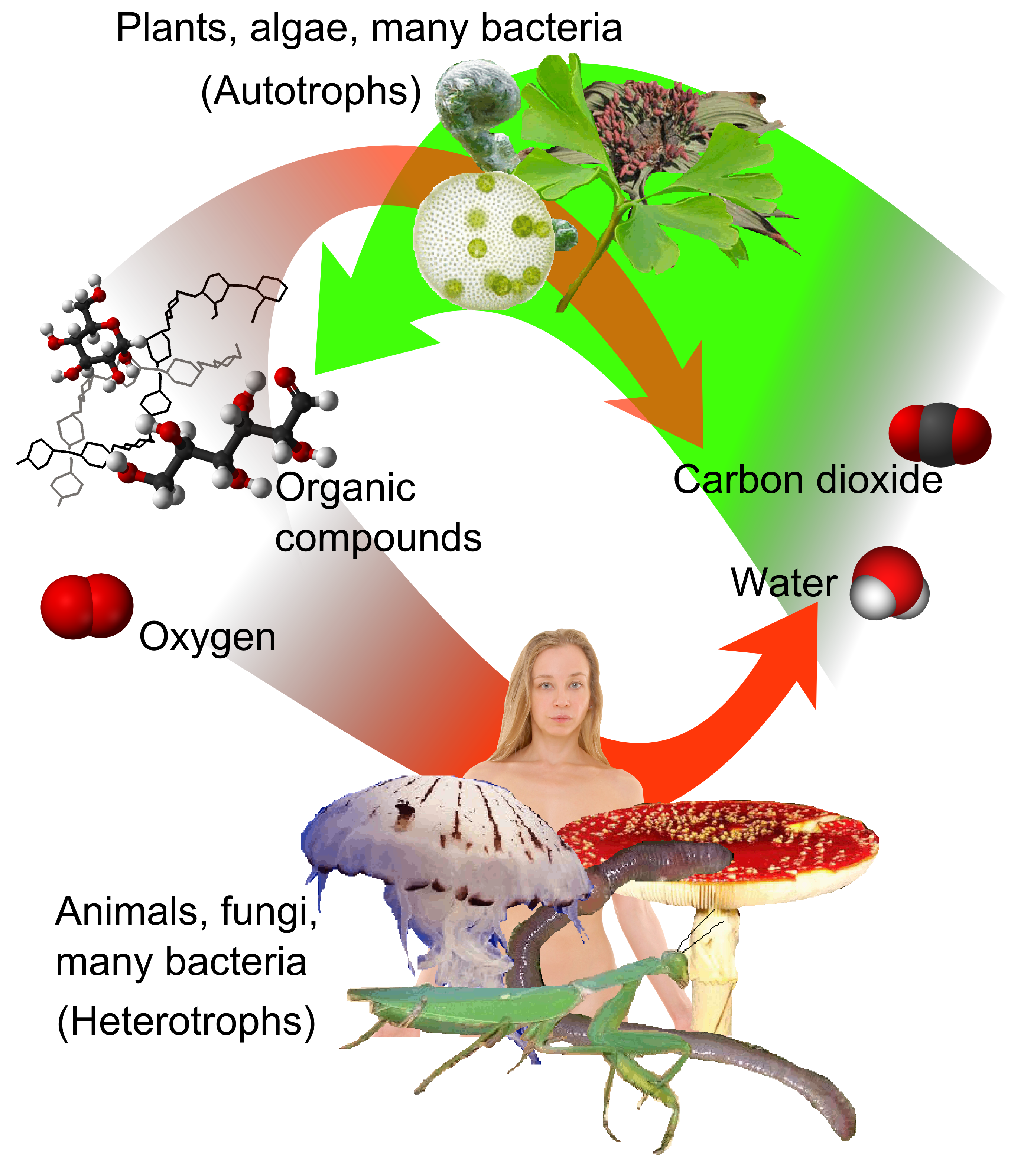
Heterotroph
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but not producers. Living organisms that are heterotrophic include all animals and fungi, some bacteria and protists, and many parasitic plants. The term heterotroph arose in microbiology in 1946 as part of a classification of microorganisms based on their type of Primary nutritional groups, nutrition. The term is now used in many fields, such as ecology, in describing the food chain. Heterotrophs occupy the second and third trophic levels of the food chain while autotrophs occupy the first trophic level. Heterotrophs may be subdivided according to their energy source. If the heterotroph uses chemical energy, it is a chemotroph, chemoheterotroph (e.g., humans and mushrooms). If it uses light for energy, then it is a photoheterotroph (e.g., gre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |