|
Diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane. Use For safety and convenience diazomethane is always prepared as needed as a solution in diethyl ether, ether and used as such. It converts carboxylic acids to methyl esters and phenols into their methyl ethers. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give a methyldiazonium cation, which reacts with the carboxylate ion to give th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazomethane Synthesis V
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost universally used as a solution in diethyl ether. The compound is a popular methylating agent in the laboratory, but it is too hazardous to be employed on an industrial scale without special precautions. Use of diazomethane has been significantly reduced by the introduction of the safer and equivalent reagent trimethylsilyldiazomethane. Use For safety and convenience diazomethane is always prepared as needed as a solution in ether and used as such. It converts carboxylic acids to methyl esters and phenols into their methyl ethers. The reaction is thought to proceed via proton transfer from carboxylic acid to diazomethane to give a methyldiazonium cation, which reacts with the carboxylate ion to give the methyl es ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylsilyldiazomethane
Trimethylsilyldiazomethane is the organosilicon compound with the formula (CH3)3SiCHN2. It is classified as a diazo compound. Trimethylsilyldiazomethane is commercially available as solutions in hexanes, DCM, and ether. It is a specialized reagent used in organic chemistry as a methylating agent for carboxylic acids. It is a safer replacement for diazomethane, which is a sensitive explosive gas, whereas trimethylsilyldiazomethane is a relatively stable liquid and thus easier to handle. Preparation Trimethylsilyldiazomethane can be prepared by treating (trimethylsilyl)methylmagnesium chloride with diphenyl phosphorazidate. An isotopically labelled variant, with 13C at the diazomethyl carbon, is also known. Reactions Trimethylsilyldiazomethane is useful for conversion of carboxylic acids to their methyl esters in high yield. The typical reaction conditions for this purpose use methanol as a cosolvent. Under these conditions, diazomethane itself is generated in situ as the acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Büchner–Curtius–Schlotterbeck Reaction
The Buchner–Curtius–Schlotterbeck reaction is the Chemical reaction, reaction of aldehydes or ketones with aliphatic diazoalkanes to form homologated ketones. It was first described by Eduard Buchner and Theodor Curtius in 1885 and later by Fritz Schlotterbeck in 1907. Two German chemists also preceded Schlotterbeck in discovery of the reaction, Hans von Pechmann in 1895 and Viktor Meyer in 1905. The reaction has since been extended to the synthesis of β-keto esters from the condensation between aldehydes and diazo esters. The general reaction scheme is as follows: The reaction yields two possible carbonyl compounds (I and II) along with an epoxide (III). The ratio of the products is determined by the reactant used and the reaction conditions. Reaction mechanism The general mechanism is shown below. The resonating arrow (1) shows a Resonance (chemistry), resonance contributor of the diazo compound with a lone pair of electrons on the carbon adjacent to the nitrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arndt–Eistert Reaction
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. It is named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978). The method entails treating an acid chloride with diazomethane. It is a popular method of producing β-amino acids from α-amino acids. Conditions Aside from the acid chloride substrate, three reagents are required: diazomethane, water, and a metal catalyst. Each has been well investigated. The diazomethane is required in excess so as to react with the HCl formed previously. Not taking diazomethane in excess results in HCl reacting with the diazoketone to form chloromethyl ketone and N2. Mild conditions allow this reaction to take place while not affecting complex or reducible groups in the reactant-acid. The reaction requires the presence of a nucleophile (water). A metal catalyst is required. Usually Ag2O is chosen but other metals and even light effect the rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazo Compound
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula . The simplest example of a diazo compound is diazomethane, . Diazo compounds () should not be confused with azo compounds () or with diazonium compounds (). Structure The electronic structure of diazo compounds is characterized by π electron density delocalized over the α-carbon and two nitrogen atoms, along with an orthogonal π system with electron density delocalized over only the terminal nitrogen atoms. Because all octet rule-satisfying resonance forms of diazo compounds have formal charges, they are members of a class of compounds known as 1,3-dipoles. Some of the most stable diazo compounds are α-diazo-β-diketones and α-diazo-β-diesters, in which the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylating Agent
Methylation, in the chemical sciences, is the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and biology. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. ''In vitro'' methylation of tissue samples is also a way to reduce some histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals and can regulate gene expression, RNA processing, and protein function. It is a key process underlying epigenetics. Sources of methyl groups include S-methylmethionine, methyl folate, methyl B12. Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyldiazonium
Methyldiazonium is an organic compound consisting of a methyl group attached to a diazo group. This cation is the conjugate acid of diazomethane, with an estimated p''K''a<10. : 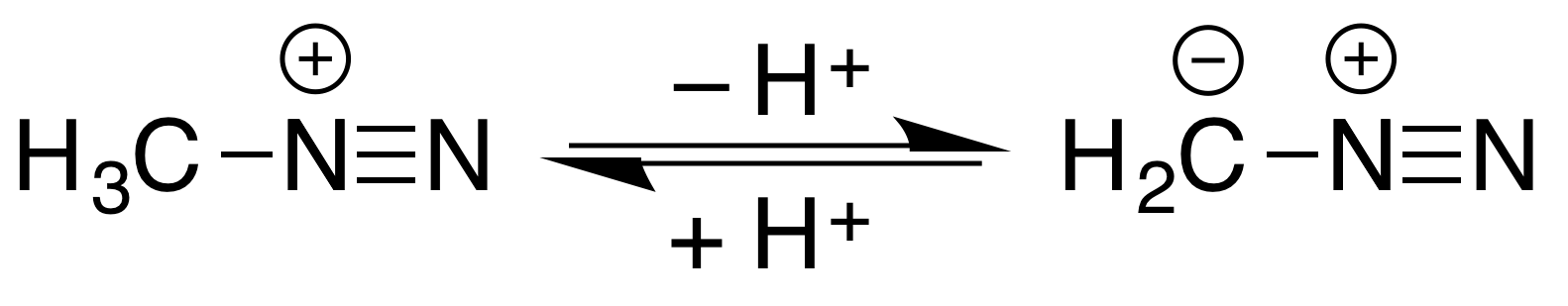 It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of s to methyl esters and
It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of s to methyl esters and
|
Diazald
Diazald (''N''-methyl-''N''-nitroso-''p''-toluenesulfonamide) is used as a relatively safe and easily handled precursor to diazomethane, which is toxic and unstable. Diazald has become the favored commercially available precursor for the synthesis of diazomethane, compared to reagents like N-Nitroso-N-methylurea, ''N''-methyl''-N''-nitrosourea and Methylnitronitrosoguanidine, ''N''-methyl-''N'''-nitro-''N''-nitrosoguanidine, which are less thermally stable and more toxic and mutagenic, respectively. Upon the addition of a Base (chemistry), base such as sodium hydroxide or potassium hydroxide and mild heating (65–70 °C) in a mixture of water, diethyl ether, and a high boiling polar cosolvent (e.g., 2-(2-Methoxyethoxy)ethanol, diethylene glycol monomethyl ether), the ''N''-nitrososulfonamide undergoes successive elimination reactions to produce diazomethane (which is codistilled as an ethereal solution) as well as a P-Toluenesulfonic acid, ''p''-toluenesulfonate salt as a byp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" may also refer to the specific compound , also called methylene radical, methylene, the parent hydride from which all other carbene compounds are formally derived. There are two types of carbenes: singlet state, singlets or triplet state, triplets, depending upon their electronic structure. The different classes undergo different reactions. Most carbenes are extremely reactive and short-lived. A small number (the diHalogen, halocarbenes, carbon monoxide, and carbon monosulfide) can be isolated, and can stabilize as Coordination complex, metal ligands, but otherwise cannot be stored in bulk. A rare exception are the persistent carbenes, which have extensive application in modern organometallic chemistry. Generatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyldiazonium
Methyldiazonium is an organic compound consisting of a methyl group attached to a diazo group. This cation is the conjugate acid of diazomethane, with an estimated p''K''a<10. :  It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of s to methyl esters and
It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of s to methyl esters and
|
Hans Von Pechmann
Hans Freiherr von Pechmann (1 April 1850 – 19 April 1902) was a German chemist, renowned for his discovery of diazomethane in 1894. Pechmann condensation and Pechmann pyrazole synthesis. He also first prepared 1,2-diketones (e.g., diacetyl), acetonedicarboxylic acid, methylglyoxal and diphenyltriketone; established the symmetrical structure of anthraquinone. Von Pechmann also produced the first example of solid polyethylene serendipitously in 1898, via the decomposition of diazomethane. Life Von Pechmann was born in Nürnberg, the only son of a doctor, who was also named Hans. The von Pechmanns had distinguished themselves as soldiers; in 1702, von Pechmann's ancestor Martin Günther von Pechmann, a general of artillery in the Bavarian army, had been raised to the rank of a baron of the Holy Roman Empire by Leopold I. After studying with Heinrich Limpricht at the University of Greifswald he became professor at the University of Munich till 1895. He was professor at the Univ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




