Büchner–Curtius–Schlotterbeck Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Buchner–Curtius–Schlotterbeck reaction is the  The reaction yields two possible
The reaction yields two possible
 The reaction is then completed either by the reformation of the carbonyl through an
The reaction is then completed either by the reformation of the carbonyl through an  The epoxide product is formed by an intramolecular
The epoxide product is formed by an intramolecular 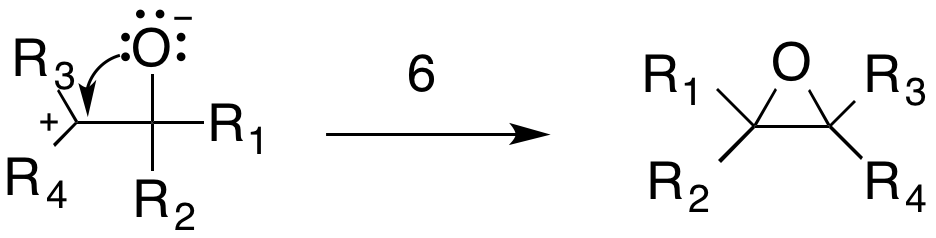 This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between
This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between
 The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.
The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.

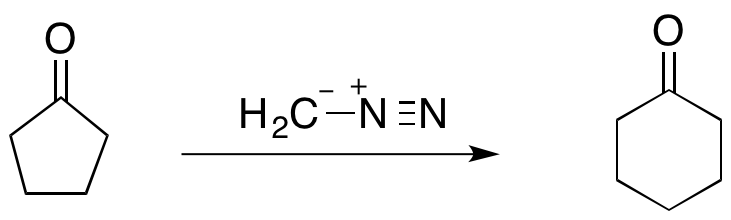 An acyl-
An acyl- Acyl-
Acyl- The Büchner–Curtius–Schlotterbeck reaction can also be used to
The Büchner–Curtius–Schlotterbeck reaction can also be used to 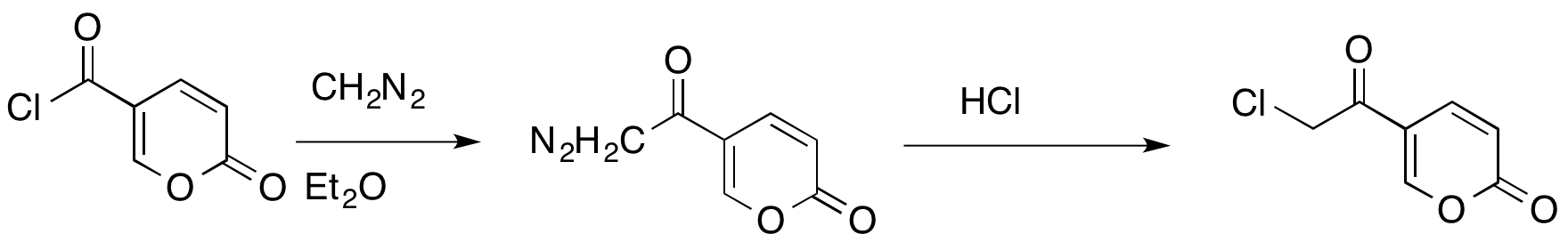 It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).
It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).

reaction
Reaction may refer to a process or to a response to an action, event, or exposure.
Physics and chemistry
*Chemical reaction
*Nuclear reaction
*Reaction (physics), as defined by Newton's third law
* Chain reaction (disambiguation)
Biology and ...
of aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
or ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
with aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all ...
diazo
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds ...
alkanes to form homologated ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
. It was first described by Eduard Buchner
Eduard Buchner (; 20 May 1860 – 13 August 1917) was a German chemist and Zymurgy, zymologist, awarded the 1907 Nobel Prize in Chemistry for his work on fermentation (biochemistry), fermentation.
Biography
Early years
Buchner was born in Mun ...
and Theodor Curtius in 1885 and later by Fritz Schlotterbeck in 1907. Two German chemists also preceded Schlotterbeck in discovery of the reaction, Hans von Pechmann
Hans Freiherr von Pechmann (1 April 1850 – 19 April 1902) was a German chemist, renowned for his discovery of diazomethane in 1894. Pechmann condensation and Pechmann pyrazole synthesis. He also first prepared 1,2-diketones (e.g., diacetyl), ...
in 1895 and Viktor Meyer
Viktor Meyer (8 September 18488 August 1897) was a German chemist and significant contributor to both organic and inorganic chemistry. He is best known for inventing an apparatus for determining vapour densities, the Viktor Meyer apparatus, and ...
in 1905. The reaction has since been extended to the synthesis of β-keto esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
from the condensation between aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
and diazo
In organic chemistry, the diazo group is an organic moiety consisting of two linked nitrogen atoms at the terminal position. Overall charge-neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds ...
esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
. The general reaction scheme is as follows:
 The reaction yields two possible
The reaction yields two possible carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
compounds (I and II) along with an epoxide
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
(III). The ratio of the products is determined by the reactant used and the reaction conditions.
Reaction mechanism
The general mechanism is shown below. The resonating arrow (1) shows a resonance contributor of the diazo compound with a lone pair ofelectrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
on the carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
adjacent to the nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
. The diazo compound then does a nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
on the carbonyl-containing compound (nucleophilic addition
In organic chemistry, a nucleophilic addition (AN) reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic addit ...
), producing a tetrahedral intermediate
A tetrahedral intermediate is a reaction intermediate in which the bond arrangement around an initially double-bonded carbon atom has been transformed from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a c ...
(2). This intermediate decomposes by the evolution of nitrogen gas forming the tertiary carbocation intermediate (3).
 The reaction is then completed either by the reformation of the carbonyl through an
The reaction is then completed either by the reformation of the carbonyl through an 1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Frank C. Whitmore, Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atom ...
or by the formation of the epoxide. There are two possible carbonyl products: one formed by migration of R1 (4) and the other by migration of R2 (5). The relative yield of each possible carbonyl is determined by the migratory preferences of the R-groups.
 The epoxide product is formed by an intramolecular
The epoxide product is formed by an intramolecular addition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
in which a lone pair from the oxygen attacks the carbocation (6).
 This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between
This reaction is exothermic due to the stability of nitrogen gas and the carbonyl containing compounds. This specific mechanism is supported by several observations. First, kinetic studies of reactions between diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
and various ketones have shown that the overall reaction follows second order kinetics. Additionally, the reactivity of two series of ketones are in the orders Cl3CCOCH3 > CH3COCH3 > C6H5COCH3 and cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
> cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Ketonic decarboxylation of adipic acid gives cyclopentanone. The reaction is conducted at elevated temperatures in t ...
> cycloheptanone
Cycloheptanone, (CH2)6CO, is a cyclic ketone also referred to as suberone. It is a colourless volatile liquid. Cycloheptanone is used as a precursor for the synthesis of pharmaceuticals.
Synthesis
In 1836, French chemist Jean-Baptiste Boussinga ...
> cyclooctanone. These orders of reactivity are the same as those observed for reactions that are well established as proceeding through nucleophilic attack on a carbonyl group.
Scope and variation
The reaction was originally carried out indiethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
and routinely generated high yields due to the inherent irreversibly of the reaction caused by the formation of nitrogen gas
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh i ...
. Though these reactions can be carried out at room temperature, the rate does increase at higher temperatures. Typically, the reaction is carried out at less than refluxing temperatures. The optimal reaction temperature is determined by the specific diazoalkane used. Reactions involving diazomethanes with alkyl or aryl substituents are exothermic at or below room temperature. Reactions involving diazomethanes with acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl grou ...
or aroyl substituents require higher temperatures. The reaction has since been modified to proceed in the presence of Lewis acids
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
and common organic solvents such as THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
and dichloromethane
Dichloromethane (DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with ...
. Reactions generally run at room temperature for about an hour, and the yield ranges from 70%-80% based on the choice of Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
and solvent.
Steric effects
Steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
of the alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
substituents on the carbonyl reactant have been shown to affect both the rates
Rate or rates may refer to:
Finance
* Rate (company), an American residential mortgage company formerly known as Guaranteed Rate
* Rates (tax), a type of taxation system in the United Kingdom used to fund local government
* Exchange rate, rate ...
and yields of Büchner–Curtius–Schlotterbeck reaction. Table 1 shows the percent yield of the ketone and epoxide products as well as the relative rates of reaction for the reactions between several methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as ...
alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
ketones and diazomethane.
The observed decrease in rate and increase in epoxide yield as the size of the alkyl group becomes larger indicates a steric effect.
Electronic effects
Ketones and aldehydes with electron-withdrawing substituents react more readily with diazoalkanes than those bearing electron-donating substituents (Table 2). In addition to accelerating the reaction, electron-withdrawing substituents typically increase the amount of epoxide produced (Table 2). The effects of substituents on the diazoalkanes is reversed relative to the carbonyl reactants: electron-withdrawing substituents decrease the rate of reaction while electron-donating substituents accelerate it. For example, diazomethane is significantly more reactive thanethyl diazoacetate
Ethyl diazoacetate (N=N=CHC(O)OC2H5) is a diazo compound and a reagent in organic chemistry. It was discovered by Theodor Curtius in 1883. The compound can be prepared by reaction of the ethyl ester of glycine with sodium nitrite and sodium aceta ...
, though less reactive than its higher alkyl homologs (e.g. diazoethane). Reaction conditions may also affect the yields of carbonyl product and epoxide product. In the reactions of ''o''-nitrobenzaldehyde, ''p''-nitrobenzaldehyde, and phenylacetaldehyde
Phenylacetaldehyde is an organic compound used in the synthesis of fragrances and polymers. Phenylacetaldehyde is an aldehyde that consists of acetaldehyde bearing a phenyl substituent; the parent member of the phenylacetaldehyde class of compound ...
with diazomethane, the ratio of epoxide to carbonyl is increased by the inclusion of methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
in the reaction mixture. The opposite influence has also been observed in the reaction of piperonal
Piperonal, also known as heliotropin, is an organic compound which is commonly found in fragrances and flavors.Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe a ...
with diazomethane, which exhibits increased carbonyl yield in the presence of methanol.
Migratory preferences
The ratio of the two possible carbonyl products (I and II) obtained is determined by the relative migratory abilities of the carbonyl substituents (R1 and R2). In general, the R-group most capable of stabilizing the partial positive charge formed during the rearrangement migrates preferentially. A prominent exception to this general rule is hydride shifting. The migratory preferences of the carbonyl R-groups can be heavily influenced by solvent and diazoalkane choice. For example, methanol has been shown to promote aryl migration. As shown below, if the reaction of piperanol (IV) with diazomethane is carried out in the absence of methanol, the ketone obtained though a hydride shift is the major product (V). If methanol is the solvent, an aryl shift occurs to form the aldehyde (VI), which cannot be isolated as it continues to react to form the ketone (VII) and the epoxide (VIII) products. The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.
The diazoalkane employed can also determine relative yields of products by influencing migratory preferences, as conveyed by the reactions of ''o''-nitropiperonal with diazomethane and diazoethane. In the reaction between ''o''-nitropiperonal (IX) and diazomethane, an aryl shift leads to production of the epoxide (X) in 9 to 1 excess of the ketone product (XI). When diazoethane is substituted for diazomethane, a hydride shift produces the ketone (XII), the only isolable product.

Examples in the literature
The Büchner–Curtius–Schlotterbeck reaction can be used to facilitate one carbon ring expansions when the substrate ketone is cyclic. For instance, the reaction ofcyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Ketonic decarboxylation of adipic acid gives cyclopentanone. The reaction is conducted at elevated temperatures in t ...
with diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
forms cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
(shown below). The Büchner ring expansion reactions utilizing diazoalkanes have proven to be synthetically useful as they can not only be used to form 5- and 6-membered rings, but also more unstable 7- and 8-membered rings.
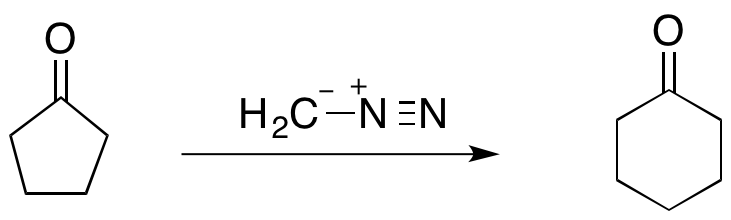 An acyl-
An acyl-diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
can react with an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
to form a β-diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls ...
in the presence of a transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
( SnCl2 in the example shown below). β-Diketones are common biological products, and as such, their synthesis is relevant to biochemical research. Furthermore, the acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
ic β-hydrogens of β-diketones are useful for broader synthetic purposes, as they can be removed by common bases.
 Acyl-
Acyl-diazomethane
Diazomethane is an organic chemical compound with the formula CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow ga ...
can also add to esters
In chemistry, an ester is a chemical compound, compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds c ...
to form β-keto esters, which are important for fatty acid synthesis
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes. Two ''De novo synthesis, de novo'' fatty acid syntheses can be distinguished: cytosolic fatty acid synthesis (FAS/FASI) ...
. As mentioned above, the acidic β-hydrogens also have productive functionality.
 The Büchner–Curtius–Schlotterbeck reaction can also be used to
The Büchner–Curtius–Schlotterbeck reaction can also be used to insert
An SQL INSERT statement adds one or more records to any single table in a relational database.
Basic form
Insert statements have the following form:
The number of columns and values must be the same. If a column is not specified, the default va ...
a methylene bridge
In chemistry, a methylene bridge is part of a molecule with formula . The carbon atom is connected by single bonds to two other distinct atoms in the rest of the molecule. A methylene bridge is often called a methylene group or simply methylene, ...
between a carbonyl carbon and a halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
of an acyl halide
An acyl halide (also known as an acid halide) is a chemical compound derived from an oxoacid by replacing a hydroxyl group () with a halide group (, where X is a halogen).
In organic chemistry, the term typically refers to acyl halides of carbox ...
. This reaction allows conservation of the carbonyl and halide functionalities.
 It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).
It is possible to isolate nitrogen-containing compounds using the Büchner–Curtius–Schlotterbeck reaction. For example, an acyl-diazomethane can react with an aldehyde in the presence of a DBU catalyst to form isolable α-diazo-β-hydroxy esters (shown below).

References
{{DEFAULTSORT:Buchner-Curtius-Schlotterbeck reaction Organic reactions Name reactions