|
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon–fluorine Bond
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry (after the B–F single bond, Si–F single bond, and H–F single bond), and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. For this reason, fluoroalkanes like tetrafluoromethane (carbon tetrafluoride) are some of the most unreactive organic compounds. Electronegativity and bond strength The high electronegativity of fluorine (4.0 for fluorine vs. 2.5 for carbon) gives the carbon–fluorine bond a significant polarity or dipole moment. The electron density is concentrated around the fluorine, leaving the carbon relatively electron poor. This introduces ionic character to the bond through partial charges (Cδ+—Fδ−). The partial charges on the fluorine and carbon ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorochemical Industry
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons per year by 2015. The largest market is the United States. Western Europe is the second largest. Asia Pacific is the fastest growing region of production. China in particular has experienced significant growth as a fluorochemical market and is becoming a producer of them as well. Fluorite mining (the main source of fluorine) was estimated in 2003 to be a $550 million industry, extracting 4.5 million tons per year. Mined fluorite is separated into two main grades, with about equal production of each. ''Acidspar'' is at least 97% CaF2; ''metspar'' is much lower purity, 60–85%. (A small amount of the intermediate, ''ceramic'', grade is also made.) Metspar is used almost exclusively for iron smelting. Acidspar is primarily converted to hydrofluoric acid (by reaction with sulfuric acid). The resultant HF is mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" or "salt maker". When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room temperature the same becomes true of groups 1 and 15, assuming white phosphorus is taken as the standard state.This could also be the case for group 12, al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important Reagent, chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
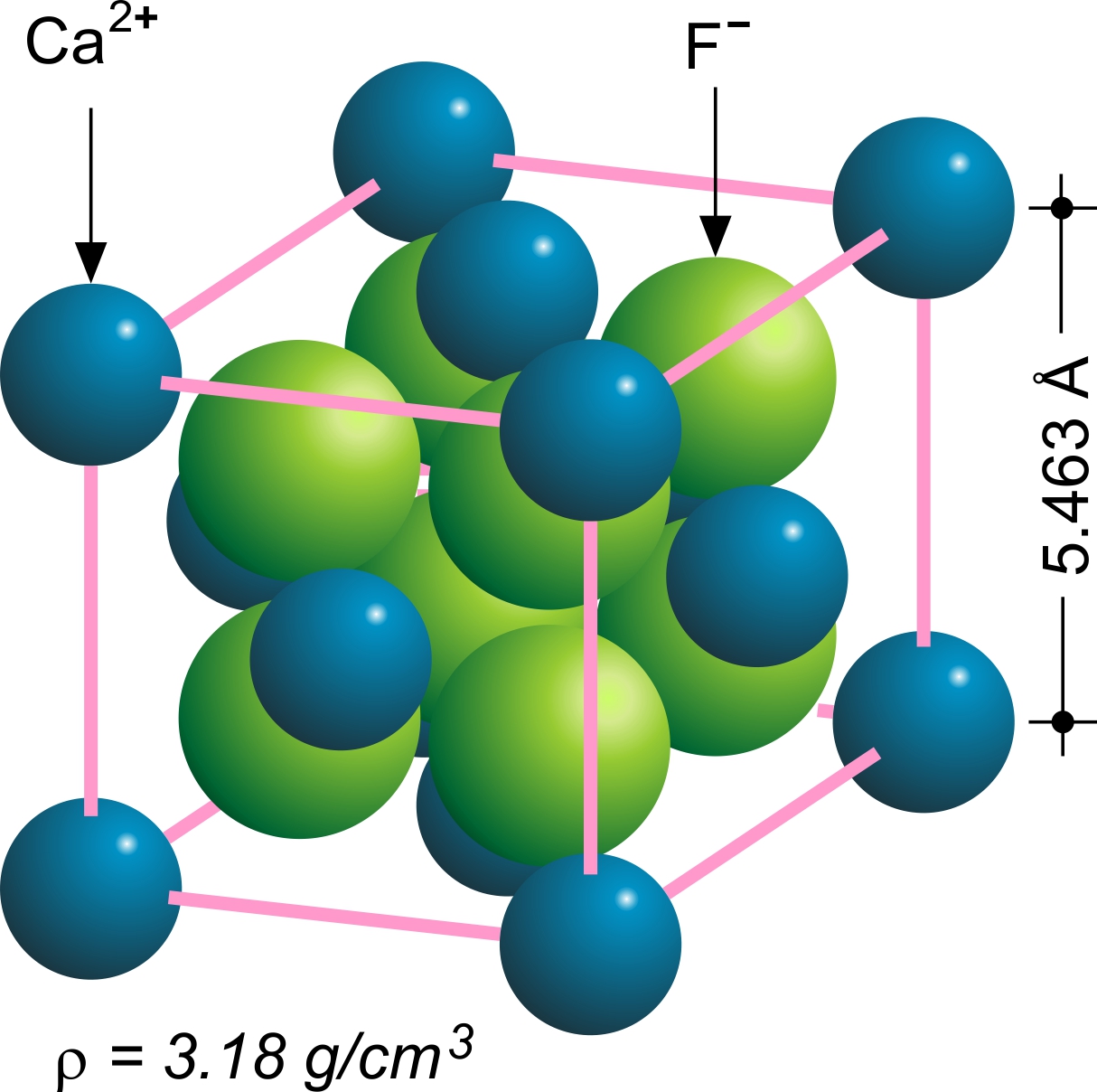
Fluorite
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the halide minerals. It crystallizes in isometric cubic habit, although octahedral and more complex isometric forms are not uncommon. The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite. Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite has anomalous partial dispersion, that is, its refractive index varies with the wavelength of light in a manner that differs from that of commonly used glasses, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henri Moissan
Ferdinand Frédéric Henri Moissan (; 28 September 1852 – 20 February 1907) was a French chemist and pharmacist who won the 1906 Nobel Prize in Chemistry for his work in isolating fluorine from its compounds. Among his other contributions, Moissan discovered moissanite and contributed to the development of the electric arc furnace. Moissan was one of the original members of the International Atomic Weights Committee. Biography Early life and education Moissan was born in Paris on 28 September 1852, the son of a minor officer of the Eastern Railway Company, Francis Ferdinand Moissan, and a seamstress, Joséphine Améraldine (née Mitel). In 1864 they moved to Meaux, where he attended the local school. During this time, Moissan became an apprentice clockmaker. However, in 1870, Moissan and his family moved back to Paris due to war against Prussia. Moissan was unable to receive the ''grade universitaire'' necessary to attend university. After spending a year in the army, he en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other sciences. It is a depiction of the periodic law, which states that when the elements are arranged in order of their atomic numbers an approximate recurrence of their properties is evident. The table is divided into four roughly rectangular areas called blocks. Elements in the same group tend to show similar chemical characteristics. Vertical, horizontal and diagonal trends characterize the periodic table. Metallic character increases going down a group and from right to left across a period. Nonmetallic character increases going from the bottom left of the periodic table to the top right. The first periodic table to become generally accepted was that of the Russian chemist Dmitri Mendeleev in 1869; he formulated the periodic law as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
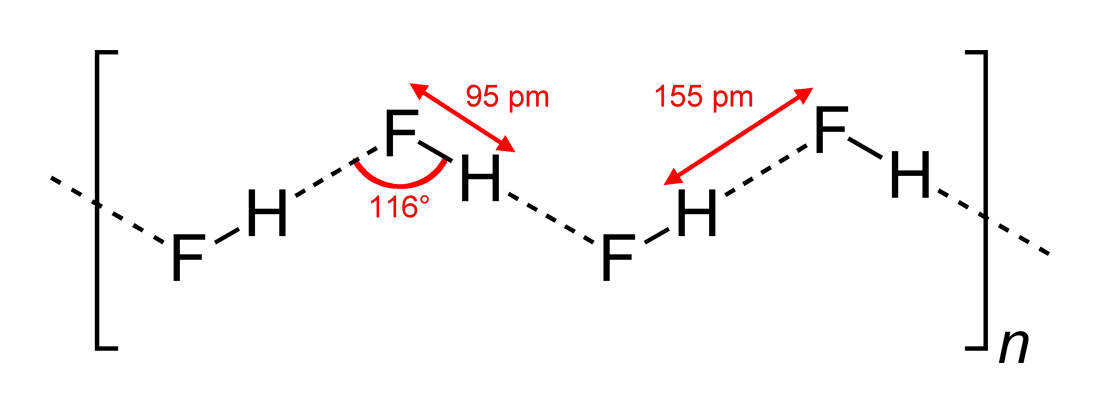
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noble Gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some cases, oganesson (Og). Under Standard temperature and pressure, standard conditions, the first six of these Chemical element, elements are odorless, colorless, monatomic gases with very low chemical reactivity and cryogenics, cryogenic boiling points. The properties of oganesson are uncertain. The intermolecular force between noble gas atoms is the very weak London dispersion force, so their boiling points are all cryogenic, below . The noble gases' Chemically inert, inertness, or tendency not to Chemical reaction, react with other chemical substances, results from their electron configuration: their Electron shell, outer shell of valence electrons is "full", giving them little tendency to participate in chemical reactions. Only a few hun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Fluoridation
Water fluoridation is the controlled addition of fluoride to Public water supply, public water supplies to reduce tooth decay. Fluoridated water maintains fluoride levels effective for cavity prevention, achieved naturally or through supplementation. In the mouth, fluoride slows tooth enamel Demineralization (physiology), demineralization and enhances remineralization in early-stage cavities. Defluoridation is necessary when natural fluoride exceeds recommended limits. The World Health Organization (WHO) recommends fluoride levels of 0.5–1.5 mg/L, depending on climate and other factors. In the U.S., the recommended level has been 0.7 mg/L since 2015, lowered from 1.2 mg/L. Bottled water often has unknown fluoride levels. Tooth decay affects 60–90% of schoolchildren worldwide. Fluoridation reduces cavities in children, with Cochrane (organisation), Cochrane reviews estimating reductions of 35% in baby teeth and 26% in permanent teeth when no other fluoride s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytetrafluoroethylene
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a corporate spin-off, spin-off from DuPont (1802–2017), DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high-molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances Wetting, wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low polarizability, electric polarizability of fluorine. PTFE has one of the lowest Friction#Coefficient of friction, coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for Cookware and bakeware, pans and other Cookware and bakeware, cookware. It is Chemically inert, non-reactive, partly because of the strength of car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of chemical element, elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity." Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek language, Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure chemical element, elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |