|
Cubane
Cubane is a synthetic hydrocarbon compound with the Chemical formula, formula . It consists of eight carbon atoms arranged at the corners of a Cube (geometry), cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Before this work, Eaton believed that cubane would be impossible to synthesize due to the "required 90 degree molecular geometry, bond angles". The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strain (chemistry), strained as compared to the tetrahedral molecular geometry#Tetrahedral bond angle, 109.45° angle of a tetrahedral geometry, tetrahedral carbon. Once formed, cubane is quite kinetic stability, kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry. Havi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octanitrocubane
Octanitrocubane (molecular formula: C8(NO2)8) is a proposed high explosive that, like TNT, is shock-insensitive (not readily detonated by shock). The octanitrocubane molecule has the same chemical structure as cubane (C8H8) except that each of the eight hydrogen atoms is replaced by a nitro group (NO2). As of 1998, octanitrocubane had not been produced in quantities large enough to test its performance as an explosive. It is, however, not as powerful an explosive as once thought, as the high-density theoretical crystal structure has not been achieved. For this reason, heptanitrocubane, the slightly less nitrated form, is believed to have marginally better performance, despite having a worse oxygen balance. Octanitrocubane is thought to have 20–25% greater performance than HMX (octogen). This increase in power is due to its highly expansive breakdown into CO2 and N2, as well as to the presence of strained chemical bonds in the molecule which have stored potential energy. In a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heptanitrocubane
Heptanitrocubane is an experimental high explosive based on the cubic eight-carbon cubane molecule and closely related to octanitrocubane. Seven of the eight hydrogen atoms at the corners of the cubane molecule are replaced by nitro groups, giving the final molecular formula . As with octanitrocubane, not enough heptanitrocubane has been synthesized to perform detailed tests on its stability and energy. It is hypothesized to have slightly better performance than explosives such as HMX, the current high-energy standard explosive, based on chemical energy analysis. While in theory not as energetic as octanitrocubane's theoretical maximum density, the heptanitrocubane that has been synthesized so far is a more effective explosive than any octanitrocubane that has been produced, due to more efficient crystal packing and hence higher density. Heptanitrocubane was first synthesized by the same team who synthesized octanitrocubane, Philip Eaton and Mao-Xi Zhang at the University o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octafluorocubane
Octafluorocubane or perfluorocubane is an organofluorine compound with the formula , consisting of eight carbon atoms joined into a cube, with a fluorine bonded to each carbon corner. It is a colorless, sublimable solid at room temperature. It has been of longstanding theoretical interest, but was not synthesised until 2022, when it was prepared in several steps from a cubane carboxylic ester beginning with its heptafluorination. According to X-ray crystallography, the C-C distances (1.570 Å) in octafluorocubane are identical in length to those in the parent cubane (1.572 Å). Octafluorocubane has attracted interest from theorists because of its unusual electronic structure, which is indicated by its susceptibility to undergo reduction to a detectable anion , with the free electron trapped inside of the cube. The compound was voted "favorite molecule of 2022" by readers of ''Chemical & Engineering News ''Chemical & Engineering News'' (''C&EN'') is a weekly news magaz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octaazacubane
Octaazacubane is a hypothetical explosive allotrope of nitrogen with formula N8, whose molecules have eight atoms arranged into a cube. (By comparison, nitrogen usually occurs as the diatomic molecule N2.) It can be regarded as a cubane-type cluster, where all eight corners are nitrogen atoms bonded along the edges. It is predicted to be a metastable molecule, in which despite the thermodynamic instability caused by bond strain, and the high energy of the N–N single bonds, the molecule remains kinetically stable for reasons of orbital symmetry. Explosive and fuel Octaazacubane is predicted to have an energy density (assuming decomposition into N2) of 22.9 MJ/ kg, which is over 5 times the standard value of TNT. It has therefore been proposed (along with other exotic nitrogen allotropes) as an explosive, and as a component of high performance rocket fuel. Its velocity of detonation is predicted to be 15,000 m/s, much (48.5%) more than octanitrocubane, the fastes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philip Eaton
Philip E. Eaton (June 2, 1936 – July 21, 2023) was an American chemist. He served as Professor Emeritus of Chemistry at the University of Chicago. Eaton and his fellow researchers were the first to synthesize the "impossible" cubane molecule in 1964.P. Eaton and T. W. Cole, The Cubane System, J. Am. Chem. Soc., 86 (1964) 962.doi:10.1021/ja01059a072/ref>P. Eaton and T. W. Cole, Cubane, J. Am. Chem. Soc., 86 (1964) 3157doi:10.1021/ja01069a041/ref> Working with Mao-Xi Zhang he is reported as having been the first to make octanitrocubane (their paper was published in the year 2003). Because of its eight nitro groups and highly strained C-C bonds, octanitrocubane is a very powerful high explosive. Early life and education Philip E. Eaton was born on June 2, 1936 in Brooklyn, New York. When Eaton was seven his family relocated to Budd Lake, New Jersey. Here he began attending Roxbury Grammar School and later Roxbury High School. It was during these high school years that he began t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cuneane
Cuneane () is a Saturated and unsaturated compounds, saturated hydrocarbon with the Chemical formula, formula and a Molecular geometry, 3D structure resembling a wedge, hence the name. Cuneane may be produced from cubane by metal-ion-catalyzed σ-bond rearrangement. Similar reactions are known for () and bishomocubane (). : Molecular geometry The carbon atoms in the cuneane molecule form a hexahedron with Molecular symmetry, point group C2v. The cuneane molecule has three kinds of equivalent carbon atoms (A, B, C), which have also been confirmed by NMR. The molecular graph of the carbon skeleton of cuneane is a regular graph with non-equivalent groups of vertices, and so it is a very important test object for different algorithms of mathematical chemistry. : Derivatives Some cuneane derivatives have liquid crystal properties. References {{Reflist Polycyclic nonaromatic hydrocarbons Cyclopropanes Cyclobutanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prismane C8
Carbon is capable of forming many allotropes (structurally different forms of the same element) due to its valency ( tetravalent). Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA). Atomic and diatomic carbon Under certain conditions, carbon can be found in its atomic form. It can be formed by vaporizing graphite, by passing large electric currents to form a carbon arc under very low pressure. It is extremely reactive, but it is an intermediate product used in the creation of carbenes. Diatomic car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Platonic Hydrocarbon
In organic chemistry, a Platonic hydrocarbon is a hydrocarbon whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed. Not all Platonic solids have molecular hydrocarbon counterparts; those that do are the tetrahedron (tetrahedrane), the cube (cubane), and the dodecahedron (dodecahedrane). The possibility and existence of each platonic hydrocarbon is affected by the number of bonds to each carbon vertex and the angle strain between the bonds at each vertex. Tetrahedrane Tetrahedrane (C4H4) is a hypothetical compound. It has not yet been synthesized without substituents, but it is predicted to be kinetically stable in spite of its angle strain. Some stable derivatives, including tetra( ''tert''-butyl)tetrahedrane and tetra(trimethylsilyl)tetrahedrane, have been produced. Cubane Cubane (C8H8) has been synthesized. Although it has high angle strain, cubane is kinet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mirex
Mirex is an organochloride that was commercialized as an insecticide and later banned because of its impact on the environment. This white crystalline odorless solid is a derivative of both cyclopentadiene and cubane. It was popularized to control fire ants but by virtue of chemical robustness and lipophilicity it was recognized as a bioaccumulative pollutant. The spread of the red imported fire ant was encouraged by the use of mirex, which also kills native ants that are highly competitive with the fire ants. The United States Environmental Protection Agency prohibited its use in 1976.Robert L. Metcalf "Insect Control" in Ullmann's Encyclopedia of Industrial Chemistry" Wiley-VCH, Wienheim, 2002. It is prohibited by the Stockholm Convention on Persistent Organic Pollutants. Production and applications Mirex was first synthesized in 1946, but was not used in pesticide formulations until 1955. Mirex was produced by the dimerization of hexachlorocyclopentadiene in the presen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
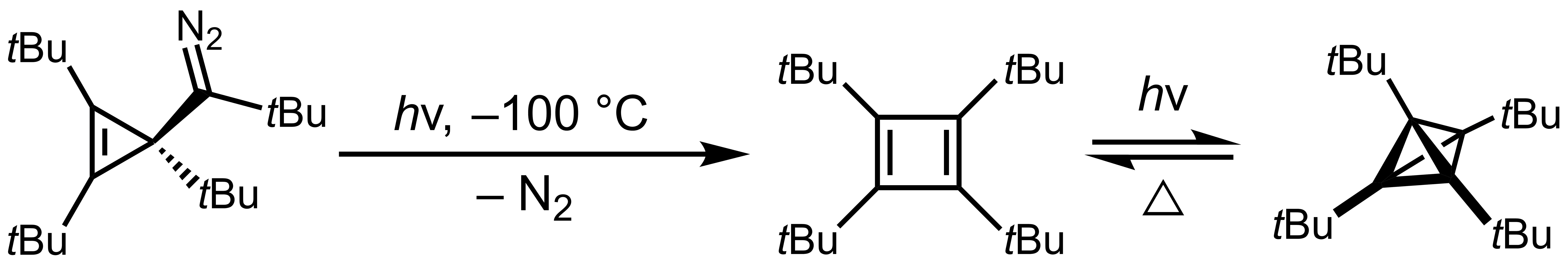
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized . However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. C4 tetrahedranes Tetrahedrane () is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane remains elusive, although predicted kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene with atomic carbon. Contrariwise, several organic compounds with the tetrahedrane core are known. All have multiply bulky substituents, ''tert''-butyl (''t''-Bu) or larger. Locking a tetrahedrane molecule inside a fullerene has only been attempted ''in silico''. All known syntheses have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cube (geometry)
A cube or regular hexahedron is a three-dimensional space, three-dimensional solid object in geometry, which is bounded by six congruent square (geometry), square faces, a type of polyhedron. It has twelve congruent edges and eight vertices. It is a type of parallelepiped, with pairs of parallel opposite faces, and more specifically a rhombohedron, with congruent edges, and a rectangular cuboid, with right angles between pairs of intersecting faces and pairs of intersecting edges. It is an example of many classes of polyhedra: Platonic solid, regular polyhedron, parallelohedron, zonohedron, and plesiohedron. The dual polyhedron of a cube is the regular octahedron. The cube can be represented in many ways, one of which is the graph known as the cubical graph. It can be constructed by using the Cartesian product of graphs. The cube is the three-dimensional hypercube, a family of polytopes also including the two-dimensional square and four-dimensional tesseract. A cube with 1, unit s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |