|
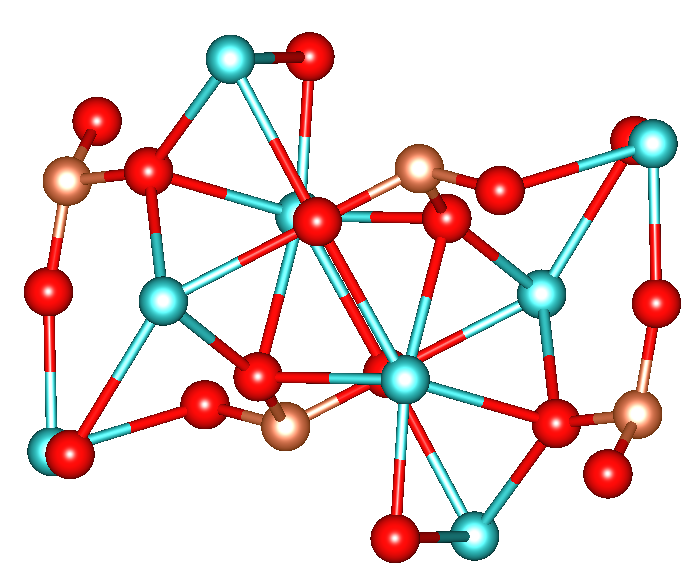
Calcium Silicate
Calcium silicate can refer to several silicates of calcium including: *CaO·SiO2, wollastonite (CaSiO3) *2CaO·SiO2, larnite (Ca2SiO4) *3CaO·SiO2, alite or (Ca3SiO5) *3CaO·2SiO2, (Ca3Si2O7). This article focuses on Ca2SiO4, also known as calcium orthosilicate, or by the shortened trade name Cal-Sil/Calsil. All calcium silicates are white free-flowing powders. Being strong, cheap and nontoxic, they are components of important structural materials. Production and occurrence Calcium silicates are produced by treating calcium oxide and silicon dioxide, silica in various ratios. Their formation is relevant to Portland cement. Calcium silicate is a byproduct of the Pidgeon process, a major route to magnesium metal. The process converts a mixture of magnesium and calcium oxides as represented by the following simplified equation: : The calcium oxide combines with silicon as the oxygen scavenger, yielding the very stable calcium silicate and releasing volatile (at high temperatures) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wollastonite
Wollastonite is a calcium Silicate minerals, inosilicate mineral (calcium, Casilicon, Sioxygen, O3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or Dolomite (rock), dolomite is subjected to high temperature and pressure, which sometimes occurs in the presence of silica-bearing fluids as in skarns or in contact with metamorphic rocks. Associated minerals include garnets, vesuvianite, diopside, tremolite, epidote, plagioclase feldspar, pyroxene and calcite. It is named after the English chemist and mineralogist William Hyde Wollaston (1766–1828). Despite its chemical similarity to the compositional spectrum of the pyroxene group of minerals—where magnesium (Mg) and iron (Fe) substitution for calcium ends with diopside and hedenbergite respectively—it is structurally very different, with a third tetrahedron in the linked chain (as opposed to two in the pyroxenes). Production trend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
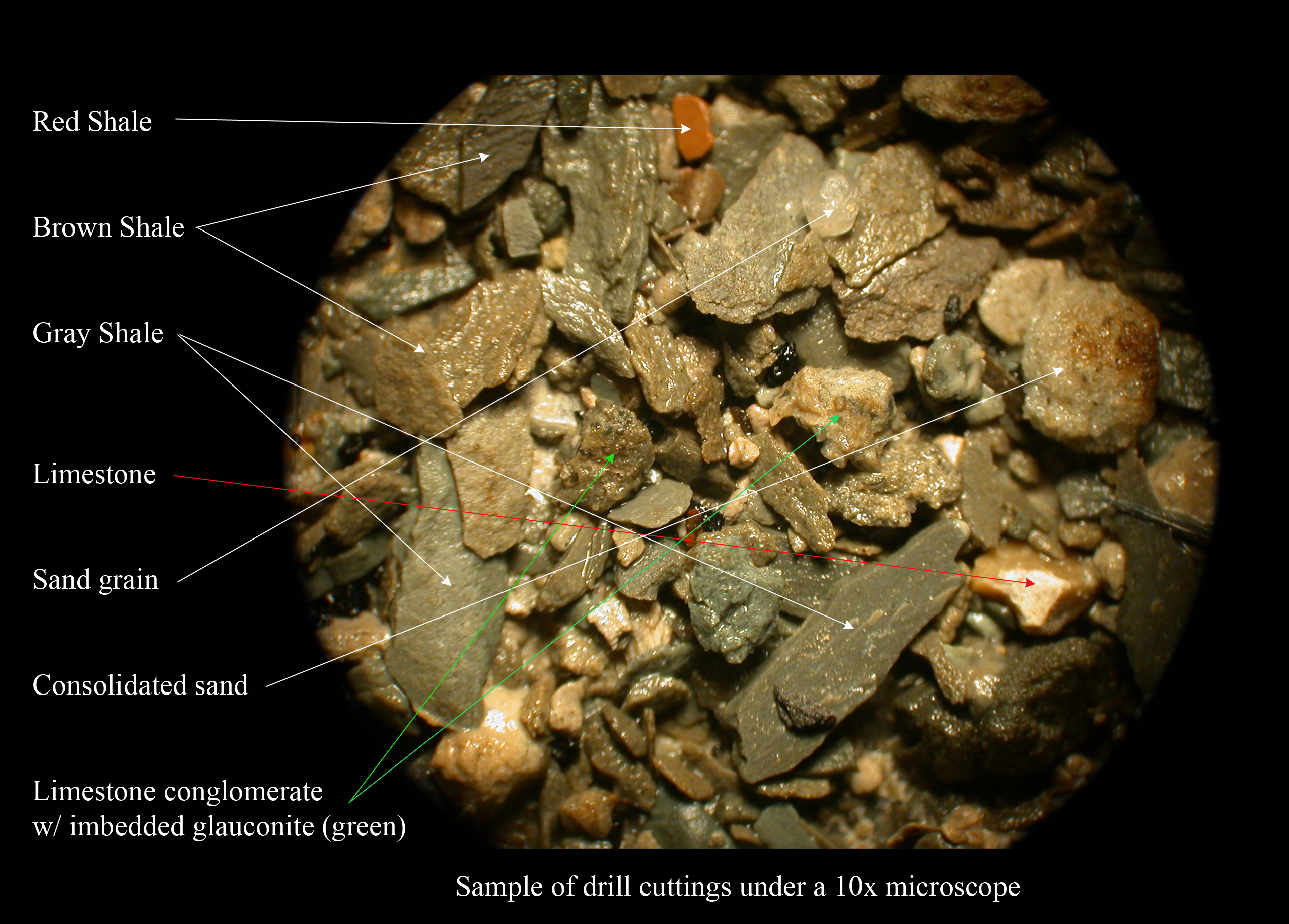
Shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of Clay mineral, clay minerals (hydrous aluminium phyllosilicates, e.g., Kaolinite, kaolin, aluminium, Al2Silicon, Si2Oxygen, O5(hydroxide, OH)4) and tiny fragments (silt-sized particles) of other minerals, especially quartz and calcite.Blatt, Harvey and Robert J. Tracy (1996) ''Petrology: Igneous, Sedimentary and Metamorphic'', 2nd ed., Freeman, pp. 281–292 Shale is characterized by its tendency to split into thin layers (Lamination (geology), laminae) less than one centimeter in thickness. This property is called ''Fissility (geology), fissility''. Shale is the most common sedimentary rock. The term ''shale'' is sometimes applied more broadly, as essentially a synonym for mudrock, rather than in the narrower sense of clay-rich fissile mudrock. Texture Shale typically exhibits varying degrees of fissility. Because of the parallel orientation of clay mineral flakes in shale, it breaks in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Silicate Cladding 2of2
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for calciu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gypsum
Gypsum is a soft sulfate mineral composed of calcium sulfate Hydrate, dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, drywall and blackboard or sidewalk chalk. Gypsum also Crystallization, crystallizes as translucent crystals of selenite (mineral), selenite. It forms as an evaporite mineral and as a Mineral hydration, hydration product of anhydrite. The Mohs scale of mineral hardness defines gypsum as hardness value 2 based on Scratch hardness, scratch hardness comparison. Fine-grained white or lightly tinted forms of gypsum known as alabaster have been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Etymology and history The word ''wikt:gypsum, gypsum'' is derived from the Greek language, Greek word (), "plaster". Because the quarry, quarries of the Montmartre district of P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Aluminoferrite
Calcium aluminoferrite () is a dark brown crystalline phase commonly found in cements. In the cement industry it is termed tetra-calcium aluminoferrite or ferrite. In cement chemist notation (CCN), it is abbreviated as meaning in the oxide notation. It also exists in nature as the rare mineral brownmillerite. Properties of the pure phase In the absence of elements other than calcium, aluminium, iron and oxygen, calcium aluminoferrite forms a solid solution series of formula for all values of x in the range 0–0.7.Taylor H.F.W. (1990). ''Cement Chemistry'', Academic Press, . Compositions with x > 0.7 do not exist at ordinary pressures (see dicalcium aluminate). The crystal is orthorhombic, and is normally lath-like. Its density varies from 4026 kg·m−3 (x = 0) to 3614 kg⋅m−3 (x = 0.7). All compositions melt incongruently in the range 1400−1450 °C. They are ferromagnetic, progressively more so as iron content increases. These phases are easily prepared ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricalcium Aluminate
Tricalcium aluminate Ca3Al2O6, often formulated as 3CaO·Al2O3 to highlight the proportions of the oxides from which it is made, is the most basic of the calcium aluminates. It does not occur in nature, but is an important mineral phase in Portland cement. Properties Tricalcium aluminate forms upon heating a 3:1 mixture of calcium oxide and aluminium oxide above 1300 °C. The crystals are cubic, with unit cell dimension 1.5263 nm and has density 3064 kg·m−3. It melts with decomposition at 1542 °C. The unit cell contains 8 cyclic Al6O1818− anions, which can be considered to consist of 6 corner sharing AlO4 tetrahedra. The structure of pure liquid tricalcium aluminate contains mostly AlO4 tetrahedra in an infinite network, with a slightly higher concentration of bridging oxygens than expected from the composition and around 10% unconnected AlO4 monomers and Al2O7 dimers. In Portland cement clinker, tricalcium aluminate occurs as an "interstitial phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Belite
Belite is an industrial mineral important in Portland cement manufacture. Its main constituent is dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2, SiO2 (C2S in cement chemist notation). Etymology The name was given by Alfred Elis Törnebohm in 1897 to a crystal identified in microscopic investigation of Portland cement. Belite is a name in common use in the cement industry, but is not a recognised mineral name. It occurs naturally as the mineral larnite, the name being derived from Larne, Northern Ireland, the closest town to Scawt Hill where it was discovered. Composition and structure The belite found in Portland cement differs in composition from pure dicalcium silicate. It is a solid solution and contains minor amounts of other oxides besides CaO and SiO2. A typical composition:Taylor H.F.W. (1990), ''Cement Chemistry'', Academic Press, 1990, , pp. 10-11. Based on this, the formula can be expressed as Ca1.94Mg0.02Na0.01K0.03Fe0.02Al0.07Si0.90P0.01O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cement Chemist Notation
Cement chemist notation (CCN) was developed to simplify the formulas cement chemists use on a daily basis. It is a shorthand way of writing the chemical formula of oxides of calcium, silicon, and various metals. Abbreviations of oxides The main oxides present in cement (or in glass and ceramics) are abbreviated in the following way: Conversion of hydroxides in oxide and free water For the sake of mass balance calculations, hydroxides present in hydrated phases found in hardened cement paste, such as in portlandite, Ca(OH)2, must first be converted into oxide and water. To better understand the conversion process of hydroxide anions in oxide and water, it is necessary to consider the autoprotolysis of the hydroxyl anions; it implies a proton exchange between two OH−, like in a classical acid–base reaction: : + → + or also, :2 OH− → O2− + H2O For portlandite this gives thus the following mass balance: :Ca(OH)2 → CaO + H2O Thus portlandite can be written as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tetrahedron is the simplest of all the ordinary convex polytope, convex polyhedra. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean geometry, Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid (geometry), pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron, the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such net (polyhedron), nets. For any tetrahedron there exists a sphere (called th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinker (cement)
image:LDClinkerScaled.jpg, 200px, Typical clinker nodules image:Hot Clinker 2.jpg, 200px, Hot clinker Cement clinker is a solid material produced in the manufacture of portland cement as an intermediary product. Clinker occurs as lumps or nodules, usually to in diameter. It is produced by sintering (fusing together without melting to the point of liquefaction) limestone and aluminosilicate materials such as clay during the cement kiln stage. Composition and preparation The Portland clinker essentially consists of four minerals: two calcium silicates, alite (Ca3SiO5) and belite (Ca2SiO4), along with tricalcium aluminate (Ca3Al2O6) and calcium aluminoferrite (Ca2(Al,Fe)2O5). These main mineral phases are produced by heating at high temperature clays and limestone. The major raw material for the clinker-making is usually limestone mixed with a second material containing clay as a source of alumino-silicate. An impure limestone containing clay or silicon dioxide (SiO2) can be used. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |