calcium silicate on:
[Wikipedia]
[Google]
[Amazon]
Calcium silicate can refer to several silicates of calcium including:
*CaO·SiO2,
/ref> :
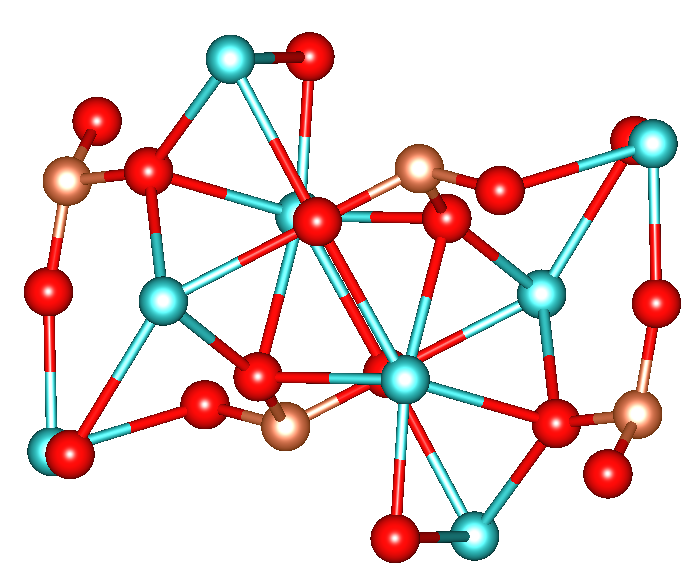 As verified by
As verified by
 Calcium silicate is commonly used as a safe alternative to
Calcium silicate is commonly used as a safe alternative to
 It is used in
It is used in
and as an
wollastonite
Wollastonite is a calcium Silicate minerals, inosilicate mineral (calcium, Casilicon, Sioxygen, O3) that may contain small amounts of iron, magnesium, and manganese substituting for calcium. It is usually white. It forms when impure limestone or D ...
(CaSiO3)
*2CaO·SiO2, larnite (Ca2SiO4)
*3CaO·SiO2, alite
Alite is an impure form of tricalcium silicate, , sometimes formulated as ( in cement chemist notation), typically with 3-4% of substituent oxides. It is the major, and characteristic, phase in Portland cement. The name was given by Alfred Elis ...
or (Ca3SiO5)
*3CaO·2SiO2, (Ca3Si2O7).
This article focuses on Ca2SiO4, also known as calcium orthosilicate
In chemistry, orthosilicate is the anion , or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.C. A. Kumins, and A. E. Gessler (1953), "Short-Cycle Syntheses of Ultr ...
, or by the shortened trade name Cal-Sil/Calsil. All calcium silicates are white free-flowing powders. Being strong, cheap and nontoxic, they are components of important structural materials.
Production and occurrence
Calcium silicates are produced by treatingcalcium oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing ...
and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
in various ratios. Their formation is relevant to Portland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in th ...
.
Calcium silicate is a byproduct of the Pidgeon process, a major route to magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
metal. The process converts a mixture of magnesium and calcium oxides as represented by the following simplified equation:
:
The calcium oxide combines with silicon as the oxygen scavenger, yielding the very stable calcium silicate and releasing volatile (at high temperatures) magnesium metal.
Via the carbonate–silicate cycle, carbonate rocks convert into silicate rocks by metamorphism
Metamorphism is the transformation of existing Rock (geology), rock (the protolith) to rock with a different mineral composition or Texture (geology), texture. Metamorphism takes place at temperatures in excess of , and often also at elevated ...
and volcanism
Volcanism, vulcanism, volcanicity, or volcanic activity is the phenomenon where solids, liquids, gases, and their mixtures erupt to the surface of a solid-surface astronomical body such as a planet or a moon. It is caused by the presence of a he ...
and silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
rocks convert to carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s by weathering
Weathering is the deterioration of rocks, soils and minerals (as well as wood and artificial materials) through contact with water, atmospheric gases, sunlight, and biological organisms. It occurs '' in situ'' (on-site, with little or no move ...
and sedimentation
Sedimentation is the deposition of sediments. It takes place when particles in suspension settle out of the fluid in which they are entrained and come to rest against a barrier. This is due to their motion through the fluid in response to th ...
.
The production of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
from anhydrous calcium sulfate produces calcium silicates. Upon being mixed with shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of Clay mineral, clay minerals (hydrous aluminium phyllosilicates, e.g., Kaolinite, kaolin, aluminium, Al2Silicon, Si2Oxygen, O5(hydroxide, OH)4) and tiny f ...
or marl
Marl is an earthy material rich in carbonate minerals, Clay minerals, clays, and silt. When Lithification, hardened into rock, this becomes marlstone. It is formed in marine or freshwater environments, often through the activities of algae.
M ...
, and roasted at 1400 °C, the sulfate liberates sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
gas, a precursor to sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
. The resulting calcium silicate is used in cement
A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mi ...
clinker production.Anhydrite Process/ref> :
Structure
 As verified by
As verified by X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
, calcium silicate is a dense solid consisting of tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
orthosilicate
In chemistry, orthosilicate is the anion , or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.C. A. Kumins, and A. E. Gessler (1953), "Short-Cycle Syntheses of Ultr ...
(SiO44-) units linked to Ca2+ via Si-O-Ca bridges. There are two calcium sites. One is seven coordinate and the other is eight coordinate.
Use
As a component of cement
Calcium silicates are the major ingredients inPortland cement
Portland cement is the most common type of cement in general use around the world as a basic ingredient of concrete, mortar (masonry), mortar, stucco, and non-specialty grout. It was developed from other types of hydraulic lime in England in th ...
s.
High-temperature insulation
 Calcium silicate is commonly used as a safe alternative to
Calcium silicate is commonly used as a safe alternative to asbestos
Asbestos ( ) is a group of naturally occurring, Toxicity, toxic, carcinogenic and fibrous silicate minerals. There are six types, all of which are composed of long and thin fibrous Crystal habit, crystals, each fibre (particulate with length su ...
for high-temperature insulation materials. Industrial-grade piping and equipment insulation is often fabricated from calcium silicate. Its fabrication is a routine part of the curriculum for insulation apprentice
Apprenticeship is a system for training a potential new practitioners of a Tradesman, trade or profession with on-the-job training and often some accompanying study. Apprenticeships may also enable practitioners to gain a license to practice in ...
s. Calcium silicate competes in these realms against rockwool and proprietary insulation solids, such as perlite
Perlite is an amorphous volcanic glass that has a relatively high water content, typically formed by the Hydrate, hydration of obsidian. It occurs naturally and has the unusual property of greatly expanding when heated sufficiently. It is an indu ...
mixture and vermiculite
Vermiculite is a hydrous phyllosilicate mineral which undergoes significant expansion when heated. Exfoliation occurs when the mineral is heated sufficiently; commercial furnaces can routinely produce this effect. Vermiculite forms by the weathe ...
bonded with sodium silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate (), sodium orthosilicate (), and sodium pyrosilicate (). The anions are often polymeric. These compounds are generally colorless tra ...
. Although it is popularly considered an asbestos substitute, early uses of calcium silicate for insulation still made use of asbestos fibers.
Passive fire protection
 It is used in
It is used in passive fire protection
Passive fire protection (PFP) is components or systems of a building or structure that slows or impedes the spread of the effects of fire or smoke without system activation, and usually without movement. Examples of passive systems include floor- ...
and fireproofing
Fireproofing is rendering something (Building, structures, materials, etc.) resistant to fire, or incombustible; or material for use in making anything fire-proof. It is a passive fire protection measure. "Fireproof" or "fireproofing" can be u ...
as calcium silicate brick or in roof tiles. It is one of the most successful materials in fireproofing
Fireproofing is rendering something (Building, structures, materials, etc.) resistant to fire, or incombustible; or material for use in making anything fire-proof. It is a passive fire protection measure. "Fireproof" or "fireproofing" can be u ...
in Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east ...
because of regulations and fire safety guidelines for commercial and residential building codes. Where North America
North America is a continent in the Northern Hemisphere, Northern and Western Hemisphere, Western hemispheres. North America is bordered to the north by the Arctic Ocean, to the east by the Atlantic Ocean, to the southeast by South Ameri ...
ns use spray fireproofing plaster
Plaster is a building material used for the protective or decorative coating of walls and ceilings and for moulding and casting decorative elements. In English, "plaster" usually means a material used for the interiors of buildings, while "re ...
s, Europeans are more likely to use cladding made of calcium silicate. High-performance calcium-silicate boards retain their excellent dimensional stability even in damp and humid conditions and can be installed at an early stage in the construction program, before wet trades are completed and the building is weather-tight. For sub-standard products, silicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
-treated sheets are available to fabricators to mitigate potential harm from high humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation (meteorology), precipitation, dew, or fog t ...
or general presence of water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
. Fabricators and installers of calcium silicate in passive fire protection
Passive fire protection (PFP) is components or systems of a building or structure that slows or impedes the spread of the effects of fire or smoke without system activation, and usually without movement. Examples of passive systems include floor- ...
often also install firestop
A firestop or fire-stopping is a form of passive fire protection that is used to seal around openings and between joints in a fire-resistance-rated wall or floor assembly. Firestops are designed to maintain the fire-resistance rating of a wall ...
s.
While the best possible reaction to fire classifications are A1 (construction applications) and A1Fl (flooring applications) respectively, both of which mean "non-combustible" according to EN 13501-1: 2007, as classified by a notified laboratory in Europe, some calcium-silicate boards only come with fire classification of A2 (limited combustibility) or even lower classifications (or no classification), if they are tested at all.
Acid mine drainage remediation
Calcium silicate, also known asslag
The general term slag may be a by-product or co-product of smelting (pyrometallurgical) ores and recycled metals depending on the type of material being produced. Slag is mainly a mixture of metal oxides and silicon dioxide. Broadly, it can be c ...
, is produced when molten iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
is made from iron ore
Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the f ...
, silicon dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundan ...
and calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
in a blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being supplied above atmospheric pressure.
In a ...
. When this material is processed into a highly refined, re-purposed calcium silicate aggregate, it is used in the remediation of acid mine drainage
Acid mine drainage, acid and metalliferous drainage (AMD), or acid rock drainage (ARD) is the outflow of acidic water from metal mines and coal mines.
Acid rock drainage occurs naturally within some environments as part of the rock weatherin ...
(AMD) on active and passive mine sites.
Calcium silicate neutralizes active acidity in AMD systems by removing free hydrogen ions from the bulk solution, thereby increasing pH. As its silicate anion captures H+ ions (raising the pH), it forms monosilicic acid (H4SiO4), a neutral solute. Monosilicic acid remains in the bulk solution to play other important roles in correcting the adverse effects of acidic conditions. As opposed to limestone (a popular remediation material), calcium silicate effectively precipitates heavy metals and does not armor over, prolonging its effectiveness in AMD systems.
As a product of sealants
It is used as a sealant in roads or on the shells of fresh eggs: whensodium silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate (), sodium orthosilicate (), and sodium pyrosilicate (). The anions are often polymeric. These compounds are generally colorless tra ...
is applied as a sealant to cured concrete
Concrete is a composite material composed of aggregate bound together with a fluid cement that cures to a solid over time. It is the second-most-used substance (after water), the most–widely used building material, and the most-manufactur ...
or egg shells, it chemically reacts with calcium hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed with water. Annually, approxim ...
or calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
to form calcium silicate hydrate, sealing micropores with a relatively impermeable material.
Agriculture
Calcium silicate is often used in agriculture as a plant available source of silicon. It is "applied extensively to Everglades mucks and associated sands planted to sugarcane and rice"Other
Calcium silicate is used as ananticaking agent
An anticaking agent is an additive placed in powdered or granulated materials, such as table salt or confectioneries, to prevent the formation of lumps ( caking) and for easing packaging, transport, flowability, and consumption. Caking mechanism ...
in food preparation, including table saltand as an
antacid
An antacid is a substance which neutralization (chemistry), neutralizes gastric acid, stomach acidity and is used to relieve heartburn, indigestion, or an upset stomach. Some antacids have been used in the treatment of constipation and diarrhe ...
. It is approved by the United Nations' FAO
The Food and Agriculture Organization of the United Nations; . (FAO) is a List of specialized agencies of the United Nations, specialized agency of the United Nations that leads international efforts to defeat hunger and improve nutrition ...
and WHO
The World Health Organization (WHO) is a specialized agency of the United Nations which coordinates responses to international public health issues and emergencies. It is headquartered in Geneva, Switzerland, and has 6 regional offices and 15 ...
bodies as a safe food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke ( smoking) and sugar ( crystallization), have been used f ...
in a large variety of products.
''Codex General Standard for Food Additives (GSFA) Online Database'', FAO/WHO Food Standards Codex alimentarius, published by the Food and Agricultural Organization of the United Nations / World Health Organization, 2013. It has the E number
E numbers, short for Europe numbers, are codes for substances used as food additives, including those found naturally in many foods, such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly ...
reference E552.
See also
* * * * * *References
{{DEFAULTSORT:Calcium Silicate Inorganic silicon compounds Calcium compounds Antacids Silicates E-number additives