|
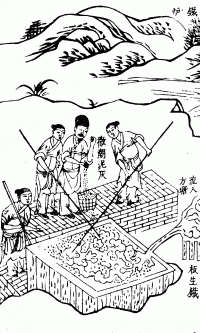
Bloomery
A bloomery is a type of metallurgical furnace once used widely for smelting iron from its iron oxides, oxides. The bloomery was the earliest form of smelter capable of smelting iron. Bloomeries produce a porous mass of iron and slag called a ''bloom''. The mix of slag and iron in the bloom, termed ''Direct reduced iron, sponge iron'', is usually consolidated and further forged into wrought iron. Blast furnaces, which produce pig iron, have largely superseded bloomeries. Process A bloomery consists of a wikt:pit, pit or chimney with heat-resistant walls made of earth, clay, or Rock (geology), stone. Near the bottom, one or more pipes (made of clay or metal) enter through the side walls. These pipes, called tuyeres, allow air to enter the furnace, either by natural draught or forced with bellows or a trompe. An opening at the bottom of the bloomery may be used to remove the bloom, or the bloomery can be tipped over and the bloom removed from the top. The first step taken b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wrought Iron
Wrought iron is an iron alloy with a very low carbon content (less than 0.05%) in contrast to that of cast iron (2.1% to 4.5%), or 0.25 for low carbon "mild" steel. Wrought iron is manufactured by heating and melting high carbon cast iron in an open charcoal or coke hearth or furnace in a process known as puddling. The high temperatures cause the excess carbon to oxidise, the iron being stirred or puddled during the process in order to achieve this. As the carbon content reduces, the melting point of the iron increases, ultimately to a level which is higher than can be achieved by the hearth, hence the wrought iron is never fully molten and many impurities remain. The primary advantage of wrought iron over cast iron is its malleability - where cast iron is too brittle to bend or shape without breaking, wrought iron is highly malleable, and much easier to bend. Wrought iron is a semi-fused mass of iron with fibrous slag inclusions (up to 2% by weight), which give it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blast Furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being supplied above atmospheric pressure. In a blast furnace, fuel ( coke), ores, and flux (limestone) are continuously supplied through the top of the furnace, while a hot blast of (sometimes oxygen enriched) air is blown into the lower section of the furnace through a series of pipes called tuyeres, so that the chemical reactions take place throughout the furnace as the material falls downward. The end products are usually molten metal and slag phases tapped from the bottom, and flue gases exiting from the top. The downward flow of the ore along with the flux in contact with an upflow of hot, carbon monoxide-rich combustion gases is a countercurrent exchange and chemical reaction process. In contrast, air furnaces (such as reverberatory furnaces) are naturally aspirated, usu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Direct Reduced Iron
Direct reduced iron (DRI), also called sponge iron, is produced from the direct reduction of iron ore (in the form of lumps, pellets, or fines) into iron by a reducing gas which contains elemental carbon (produced from natural gas or coal) and/or hydrogen. When hydrogen is used as the reducing gas no carbon dioxide is produced. Many ores are suitable for direct reduction. Direct reduction refers to solid-state processes which reduce iron oxides to metallic iron at temperatures below the melting point of iron. Reduced iron derives its name from these processes, one example being heating iron ore in a furnace at a high temperature of in the presence of syngas (a mixture of hydrogen and carbon monoxide) or pure hydrogen. Process Direct reduction processes can be divided roughly into two categories: gas-based and coal-based. In both cases, the objective of the process is to remove the oxygen contained in various forms of iron ore (sized ore, concentrates, pellets, mill scale, fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trompe
A trompe is a water-powered air compressor, commonly used before the advent of the electric-powered compressor. A trompe is somewhat like an airlift pump working in reverse. Trompes were used to provide compressed air for bloomery furnaces in Catalonia and the United States. The presence of a trompe is a signature attribute of a Catalan forge, a type of bloomery furnace. Trompes can be enormous. At Canadian Hydro Developers' Ragged Chute facility in New Liskeard, Ontario, water falls down a shaft deep and across to generate compressed air for mining equipment and ventilation.Ragged Chutes Operation Trompes are very simple devices. They consist of four main parts: a ''water-supply pipe'' or ''shaft'' with an ''air-inlet'' inside it, a ''water ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smelter
Smelting is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product. It is a form of extractive metallurgy that is used to obtain many metals such as iron, copper, silver, tin, lead and zinc. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gases or slag and leaving the metal behind. The reducing agent is commonly a fossil-fuel source of carbon, such as carbon monoxide from incomplete combustion of coke—or, in earlier times, of charcoal. The oxygen in the ore binds to carbon at high temperatures, as the chemical potential energy of the bonds in carbon dioxide () is lower than that of the bonds in the ore. Sulfide ores such as those commonly used to obtain copper, zinc or lead, are roasted before smelting in order to convert the sulfides to oxides, which are more readily reduced to the metal. Roasting heats the ore in the presence of oxygen from air, oxidizing the or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smelting
Smelting is a process of applying heat and a chemical reducing agent to an ore to extract a desired base metal product. It is a form of extractive metallurgy that is used to obtain many metals such as iron-making, iron, copper extraction, copper, silver mining#Ore processing, silver, tin, lead smelting, lead and zinc smelting, zinc. Smelting uses heat and a chemical reducing agent to decompose the ore, driving off other elements as gases or slag and leaving the metal behind. The reducing agent is commonly a fossil-fuel source of carbon, such as carbon monoxide from incomplete combustion of coke (fuel), coke—or, in earlier times, of charcoal. The oxygen in the ore binds to carbon at high temperatures, as the Chemical energy, chemical potential energy of the bonds in carbon dioxide () is lower than that of the bonds in the ore. Sulfide ores such as those commonly used to obtain copper, zinc or lead, are roasting (metallurgy), roasted before smelting in order to convert the sulfid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fayalite
Fayalite (, commonly abbreviated to Fa) is the iron-rich endmember, end-member of the olivine solid solution, solid-solution series. In common with all minerals in the olivine, olivine group, fayalite crystallizes in the orthorhombic system (space group ''Pbnm'') with cell parameters ''a'' = 4.82 Å, ''b'' = 10.48 Å and ''c'' = 6.09 Å. Fayalite forms solid solution series with the magnesium olivine endmember forsterite (Mg2SiO4) and also with the manganese rich olivine endmember tephroite (Mn2SiO4). Iron rich olivine is a relatively common constituent of acidic and Alkaline earth metal, alkaline igneous rocks such as volcanic obsidians, rhyolites, trachytes and phonolites and plutonic quartz syenites where it is associated with amphiboles. Its main occurrence is in ultramafic volcanic and plutonic rocks and less commonly in felsic plutonic rocks and rarely in granite pegmatite. It also occurs in lithophysae in obsidian. It also occurs in medium-grade thermall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science), crystal forms of calcium carbonate . Limestone forms when these minerals Precipitation (chemistry), precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly Dolomite (rock), dolomite, a closely related rock, which contains a high percentage of the mineral Dolomite (mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon is a significant element that is essential for several physiological and metabolic processes in plants. Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating. Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flux (metallurgy)
In metallurgy, a flux is a chemical reducing agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining. Some of the earliest known fluxes were sodium carbonate, potash, charcoal, coke, borax, lime, lead sulfide and certain minerals containing phosphorus. Iron ore was also used as a flux in the smelting of copper. These agents served various functions, the simplest being a reducing agent, which prevented oxides from forming on the surface of the molten metal, while others absorbed impurities into slag, which could be scraped off molten metal. Fluxes are also used in foundries for removing impurities from molten nonferrous metals such as aluminium, or for adding desirable trace elements such as titanium. As reducing agents, fluxes facilitate soldering, brazing, and welding by removing oxidation from the metals to be joined. In some applications molten flux also serves as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Forging
Forging is a manufacturing process involving the shaping of metal using localized compression (physics), compressive forces. The blows are delivered with a hammer (often a power hammer) or a die (manufacturing), die. Forging is often classified according to the temperature at which it is performed: cold forging (a type of cold working), warm forging, or hot forging (a type of hot working). For the latter two, the metal is heated, usually in a forge. Forged parts can range in weight from less than a kilogram to hundreds of metric tons.Degarmo, p. 389 Forging has been done by metalsmith, smiths for millennia; the traditional products were kitchenware, household hardware, hardware, hand tools, edged weapons, cymbals, and jewellery. Since the Industrial Revolution, forged parts are widely used in mechanism (engineering), mechanisms and machines wherever a component requires high strength of materials, strength; such forgings usually require further processing (such as machining) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |