|
Bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three fluorocarbonate minerals, which includes bastnäsite-(cerium, Ce) with a formula of (Ce, La)CO3F, bastnäsite-(lanthanum, La) with a formula of (La, Ce)CO3F, and bastnäsite-(yttrium, Y) with a formula of (Y, Ce)CO3F. Some of the bastnäsites contain OH− instead of F− and receive the name of hydroxylbastnasite. Most bastnäsite is bastnäsite-(Ce), and cerium is by far the most common of the rare earths in this class of minerals. Bastnäsite and the phosphate mineral monazite are the two largest sources of cerium and other rare-earth elements. Bastnäsite was first described by the Swedish chemist Wilhelm Hisinger in 1838. It is named for the Bastnäs mine near Riddarhyttan, Västmanland, Sweden. Bastnäsite also occurs as very high-quality specimens at the Zagi Mountains, Pakistan. Bastnäsite occurs in alkali granite and syenite and in associated pegmatites. It also occurs in carbonatites and in ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbonate
A carbonate fluoride, fluoride carbonate, fluorocarbonate or fluocarbonate is a double salt containing both carbonate and fluoride. The salts are usually insoluble in water, and can have more than one kind of metal cation to make more complex compounds. Rare-earth fluorocarbonates are particularly important as ore minerals for the light rare-earth elements lanthanum, cerium and neodymium. Bastnäsite is the most important source of these elements. Other artificial compounds are under investigation as non-linear optical materials and for transparency in the ultraviolet, with effects over a dozen times greater than Potassium dideuterium phosphate. Related to this there are also chlorocarbonates and bromocarbonates. Along with these fluorocarbonates form the larger family of halocarbonates. In turn halocarbonates are a part of mixed anion materials. Compounds where fluorine connects to carbon making acids are unstable, fluoroformic acid decomposes to carbon dioxide and hydrogen fluori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
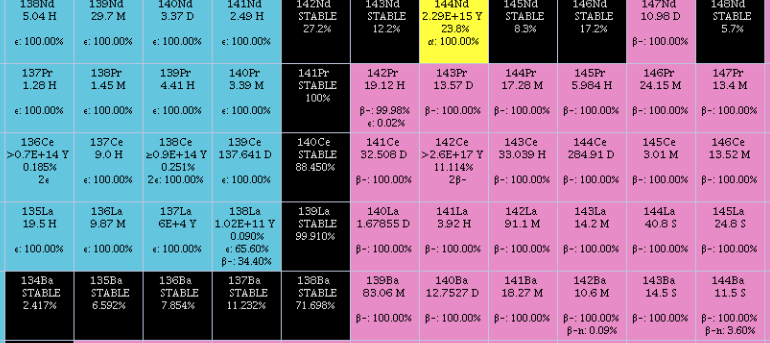
Neodymium
Neodymium is a chemical element; it has Symbol (chemistry), symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth element, rare-earth metals. It is a hard (physics), hard, slightly malleable, silvery metal that quickly tarnishes in air and moisture. When oxidized, neodymium reacts quickly producing pink, purple/blue and yellow compounds in the +2, +3 and +4 oxidation states. It is generally regarded as having one of the most complex emission spectrum, spectra of the elements. Neodymium was discovered in 1885 by the Austrian chemist Carl Auer von Welsbach, who also discovered praseodymium. Neodymium is present in significant quantities in the minerals monazite and bastnäsite. Neodymium is not found naturally in metallic form or unmixed with other lanthanides, and it is usually refined for general use. Neodymium is fairly common—about as common as cobalt, nickel, or copper—and is Abundance of elements in Eart ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the oxidation state of +3 characteristic of the series, it also has a stable +4 state that does not oxidize water. It is considered one of the rare-earth elements. Cerium has no known biological role in humans but is not particularly toxic, except with intense or continued exposure. Despite always occurring in combination with the other rare-earth elements in minerals such as those of the monazite and bastnäsite groups, cerium is easy to extract from its ores, as it can be distinguished among the lanthanides by its unique ability to be oxidized to the +4 state in aqueous solution. It is the most common of the lanthanides, followed by neodymium, lanthanum, and praseodymium. Its estimated abundance of elements in Earth's crust, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust (cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals and is never found in nature as a free element. 89Y is the only stable isotope and the only isotope found in the Crust (geology), Earth's crust. The most important present-day use of yttrium is as a component of phosphors, especially those used in LEDs. Historically, it was once widely used in the red phosphors in television set cathode ray tube displays. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers, superconductors, various medical applications, and Trace element, tracing various materials to enhance their properties. Yttrium has no known Biology, biological role. Exposure to yttrium compounds can cause Respiratory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum
Lanthanum is a chemical element; it has symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, its usual oxidation state is +3, although some compounds are known with an oxidation state of +2. Lanthanum has no biological role in humans but is used by some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the ancient Greek (), meaning 'to lie hidden'. Although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bastnäs
Bastnäs ( or ) is an ore field near Riddarhyttan, Västmanland, Sweden. The mines in Bastnäs were earliest mentioned in 1692. Iron, copper and rare-earth elements were extracted from the mines and 4,500 tons of cerium was produced between 1875 and 1888. The chemical element cerium was first discovered in Bastnäs in 1803 by Jöns Jakob Berzelius and Wilhelm Hisinger in the form of its oxide, ceria, and independently in Germany by Martin Heinrich Klaproth. Lanthanum was also first discovered in minerals from Bastnäs in 1839 by Carl Gustav Mosander. The mineral bastnäsite The mineral bastnäsite (or bastnaesite) is one of a family of three fluorocarbonate minerals, which includes bastnäsite-(cerium, Ce) with a formula of (Ce, La)CO3F, bastnäsite-(lanthanum, La) with a formula of (La, Ce)CO3F, and bastnäsite-(yt ... is named after Bastnäs. Gallery File:Bastnas maskinhus.jpg, Transmission house at Bastnäs mines File:Bastnas hakspel.jpg, Mechanism for hauling ore at Bastn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral: * monazite-(Ce), (the most common member), * monazite-(La), , * monazite-(Nd), , * monazite-(Sm), , * monazite-(Pr), . The elements in parentheses are listed in the order of their relative proportion within the mineral: lanthanum is the most common rare-earth element in monazite-(La), and so forth. Silica () is present in trace amounts, as well as small amounts of uranium and thorium. Due to the alpha decay o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fenite
Fenite is a metasomatic alteration associated particularly with carbonatite intrusions and created, very rarely, by advanced carbon dioxide alteration (carbonation) of felsic and mafic rocks. It is characterised by the presence of alkali feldspar, sodic pyroxene and sodic amphibole. Fenite alteration is known, but restricted in distribution, around high-temperature metamorphic talc carbonates, generally in the form of an aureole around ultramafic rocks. Such examples include biotite-rich zones, amphibolite-calcite-scapolite alteration and other unusual skarn assemblages. The process is called fenitization. The type locality for fenite is the Fen Complex (Norwegian: Fensfeltet) in Nome, Telemark, Norway Norway, officially the Kingdom of Norway, is a Nordic countries, Nordic country located on the Scandinavian Peninsula in Northern Europe. The remote Arctic island of Jan Mayen and the archipelago of Svalbard also form part of the Kingdom of .... References External li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metasomatism
Metasomatism (from the Greek μετά ''metá'' "change" and σῶμα ''sôma'' "body") is the chemical alteration of a rock by hydrothermal and other fluids. It is traditionally defined as metamorphism which involves a change in the chemical composition, excluding volatile components. It is the replacement of one rock by another of different mineralogical and chemical composition. The minerals which compose the rocks are dissolved and new mineral formations are deposited in their place. Dissolution and deposition occur simultaneously and the rock remains solid. Synonyms of the word ''metasomatism'' are metasomatosis and metasomatic process. The word ''metasomatose'' can be used as a name for specific varieties of metasomatism (for example '' Mg-metasomatose'' and '' Na-metasomatose''). Metasomatism can occur via the action of hydrothermal fluids from an igneous or metamorphic source. In the igneous environment, metasomatism produces skarns, greisen, and may affect hornfel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
Phosphates are the naturally occurring form of the element phosphorus. In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid, phosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one proton gives the dihydrogen phosphate ion while removal of two protons gives the hydrogen phosphate ion . These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate or orthophosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |