|
Activity Coefficient
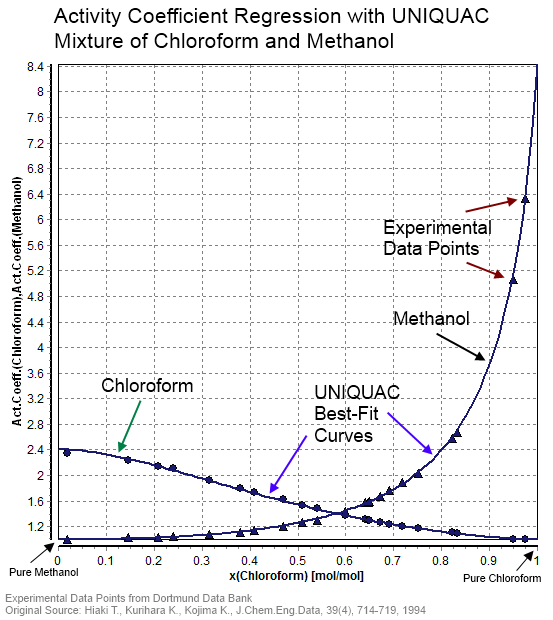
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the efficiency of early steam engines, particularly through the work of French physicist Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win the Napoleonic Wars. Scots-Irish physicist Lord Kelvin was the firs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outcome of a particular chemical change, or vice versa. These reactions involve electrons moving via an electronically-conducting phase (typically an external electrical circuit, but not necessarily, as in electroless plating) between electrodes separated by an ionically conducting and electronically insulating electrolyte (or ionic species in a solution). When a chemical reaction is driven by an electrical potential difference, as in electrolysis, or if a potential difference results from a chemical reaction as in an electric battery or fuel cell, it is called an ''electrochemical'' reaction. Unlike in other chemical reactions, in electrochemical reactions electrons are not transferred directly between atoms, ions, or molecules, but via ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colligative
In chemistry, colligative properties are those properties of solutions that depend on the ratio of the number of solute particles to the number of solvent particles in a solution, and not on the nature of the chemical species present. The number ratio can be related to the various units for concentration of a solution such as molarity, molality, normality (chemistry), etc. The assumption that solution properties are independent of nature of solute particles is exact only for ideal solutions, which are solutions that exhibit thermodynamic properties analogous to those of an ideal gas, and is approximate for dilute real solutions. In other words, colligative properties are a set of solution properties that can be reasonably approximated by the assumption that the solution is ideal. Only properties which result from the dissolution of a nonvolatile solute in a volatile liquid solvent are considered.KL Kapoor ''Applications of Thermodynamics'' Volume 3 They are essentially solvent pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Solution Chemistry
A journal, from the Old French ''journal'' (meaning "daily"), may refer to: *Bullet journal, a method of personal organization *Diary, a record of what happened over the course of a day or other period *Daybook, also known as a general journal, a daily record of financial transactions *Logbook, a record of events important to the operation of a vehicle, facility, or otherwise *Record (other) *Transaction log, a chronological record of data processing *Travel journal In publishing, ''journal'' can refer to various periodicals or serials: *Academic journal, an academic or scholarly periodical **Scientific journal, an academic journal focusing on science **Medical journal, an academic journal focusing on medicine **Law review, a professional journal focusing on legal interpretation *Magazine, non-academic or scholarly periodicals in general **Trade magazine, a magazine of interest to those of a particular profession or trade **Literary magazine, a magazine devoted to litera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angewandte Chemie
''Angewandte Chemie'' (, meaning "Applied Chemistry") is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society (Gesellschaft Deutscher Chemiker). Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. According to the ''Journal Citation Reports'', the journal had a 2021 impact factor of 16.823. Editions The journal appears in two editions with separate volume and page numbering: a German edition, ''Angewandte Chemie'' ( (print), (online)), and a fully English-language edition, ''Angewandte Chemie International Edition'' ( (print), (online)). The editions are identical in content with the exception of occasional reviews of German-language books or German translations of IUPAC recommendations. Business model ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apparent Molar Property
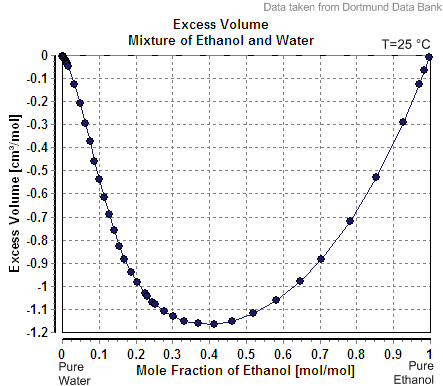
In thermodynamics, an apparent molar property of a solution component in a mixture or solution is a quantity defined with the purpose of isolating the contribution of each component to the non-ideality of the mixture. It shows the change in the corresponding solution property (for example, volume) per mole of that component added, when all of that component is added to the solution. It is described as ''apparent'' because it appears to represent the molar property of that component ''in solution'', provided that the properties of the other solution components are assumed to remain constant during the addition. However this assumption is often not justified, since the values of apparent molar properties of a component may be quite different from its molar properties in the pure state. For instance, the volume of a solution containing two components identified as solvent and solute is given by : V=V_0 + ^\phi_1 \ =\tilde_ n_ + ^\phi\tilde_1 n_1 \, where is the volume of the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvation Shell
A solvation shell or solvation sheath is the solvent interface of any chemical compound or biomolecule that constitutes the solute. When the solvent is water it is called a hydration shell or hydration sphere. The number of solvent molecules surrounding each unit of solute is called the hydration number of the solute. A classic example is when water molecules arrange around a metal ion. If the metal ion is a cation, the electronegative oxygen atom of the water molecule would be attracted electrostatically to the positive charge on the metal ion. The result is a solvation shell of water molecules that surround the ion. This shell can be several molecules thick, dependent upon the charge of the ion, its distribution and spatial dimensions. A number of molecules of solvent are involved in the solvation shell around anions and cations from a dissolved salt in a solvent. Metal ions in aqueous solutions form metal aquo complexes. This number can be determined by various methods like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye–Hückel Equation
The chemists Peter Debye and Erich Hückel noticed that solutions that contain ionic solutes do not behave ideally even at very low concentrations. So, while the concentration of the solutes is fundamental to the calculation of the dynamics of a solution, they theorized that an extra factor that they termed gamma is necessary to the calculation of the activity coefficients of the solution. Hence they developed the Debye–Hückel equation and Debye–Hückel limiting law. The activity is only proportional to the concentration and is altered by a factor known as the activity coefficient \gamma. This factor takes into account the interaction energy of ions in solution. Debye–Hückel limiting law In order to calculate the activity a_C of an ion C in a solution, one must know the concentration and the activity coefficient: a_C = \gamma \frac\mathrm\mathrm, where * \gamma is the activity coefficient of C, * \mathrm is the concentration of the chosen ''standard state'', e.g. 1 mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tacit Assumption
A tacit assumption or implicit assumption is an assumption that underlies a logical argument, course of action, decision, or judgment that is not explicitly voiced nor necessarily understood by the decision maker or judge. These assumptions may be made based on personal life experiences, and are not consciously apparent in the decision making environment. These assumptions can be the source of apparent paradoxes, misunderstandings and resistance to change in human organizational behavior. See also * Assumption-based planning * Consensus reality * Hidden curriculum * Implicit attitude * Implicit cognition * Implicit leadership theory Implicit leadership theory (ILT) is a cognitive theory of leadership developed by Robert Lord and colleagues.Forsyth, D. R. (2009). ''Group dynamics.'' New York, New York: Wadsworth. It is based on the idea that individuals create cognitive repres ... * Implicit memory * Implied consent * Leading question * Premise * Presupposition * Sha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as '' table salt'') is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather. Uses In addition to the familiar domestic uses of salt, more dominant applications of the approximately 250 million tonnes per year product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolytes
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |