|
Anhydrous Ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula . A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pungent smell. It is widely used in fertilizers, refrigerants, explosives, cleaning agents, and is a precursor for numeous chemicals. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many chemicals. In many countries, it is classified as an extremely hazardous substance. Ammonia is toxic, causing damage to cells and tissues. For this reason it is excreted by most animals in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia (data Page)
This page provides supplementary chemical data on ammonia. Structure and properties Thermodynamic properties Vapor–liquid equilibrium data Table data (above) obtained from ''CRC Handbook of Chemistry and Physics'' 44th ed. The (s) notation indicates equilibrium temperature of vapor over solid. Otherwise temperature is equilibrium of vapor over liquid. Vapor-pressure formula for ammonia:Lange's Handbook of Chemistry, 10th ed. page 1436. : log10''P'' = ''A'' – ''B'' / (''T'' − ''C''), where ''P'' is pressure in k Pa, and ''T'' is temperature in kelvins; : ''A'' = 6.67956, ''B'' = 1002.711, ''C'' = 25.215 for ''T'' = 190 K through 333 K. Heat capacity of liquid and vapor --> obtained from CHERIC. , - , , - Spectral data Regulatory data Safety data sheet The handling of this chemical may incur notable safety precautions... It is highly recommend that you seek the Safety Data Sheet ( safety data sheet, SDS) for this chemical from a reliable source an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3−''x''R''x'', where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties In its standard state arsine is a colorless, denser-than-air gas that is slightly soluble in water (2% at 20 °C) and in many organic solvents as well. Arsine itself is odorless, but it oxidizes in air and this creates a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 230 °C, decomp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the cellular metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. ''Urea'' is Neo-Latin, , , itself from Proto-Indo-European ''*h₂worsom''. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor base (chemistry), alkaline. The body uses it in many processes, most notably metabolic waste#Nitrogen wastes, nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fertiliser
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrition, plant nutrients. Fertilizers may be distinct from Liming (soil), liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and Agrochemical, industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment, or hand-tool methods. Historically, fertilization came from natural or organic sources: compost, Manure, animal manure, Human waste, human manure, harvested minerals, crop rotations, and byproducts of human-nature industries (e.g. Fish meal, fish processing waste, or B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nutrition
Nutrition is the biochemistry, biochemical and physiology, physiological process by which an organism uses food and water to support its life. The intake of these substances provides organisms with nutrients (divided into Macronutrient, macro- and Micronutrient, micro-) which can be Metabolism, metabolized to create Food energy, energy and chemical structures; too much or too little of an essential nutrient can cause malnutrition. Nutritional science, the study of nutrition as a hard science, typically emphasizes human nutrition. The type of organism determines what nutrients it needs and how it obtains them. Organisms obtain nutrients by consuming organic matter, consuming inorganic matter, absorbing light, or some combination of these. Some can produce nutrients internally by consuming basic elements, while some must consume other organisms to obtain pre-existing nutrients. All forms of life require carbon, Biological thermodynamics, energy, and water as well as various other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogenous Waste
Metabolic wastes or excrements are substances left over from metabolic processes (such as cellular respiration) which cannot be used by the organism (they are surplus or toxic), and must therefore be excreted. This includes nitrogen compounds, water, CO2, phosphates, sulphates, etc. Animals treat these compounds as excretes. Plants have metabolic pathways which transforms some of them (primarily the oxygen compounds) into useful substances. All the metabolic wastes are excreted in a form of water solutes through the excretory organs ( nephridia, Malpighian tubules, kidneys), with the exception of CO2, which is excreted together with the water vapor throughout the lungs. The elimination of these compounds enables the chemical homeostasis of the organism. Nitrogen wastes The nitrogen compounds through which excess nitrogen is eliminated from organisms are called nitrogenous wastes () or nitrogen wastes. They are ammonia, urea, uric acid, and creatinine. All of these subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
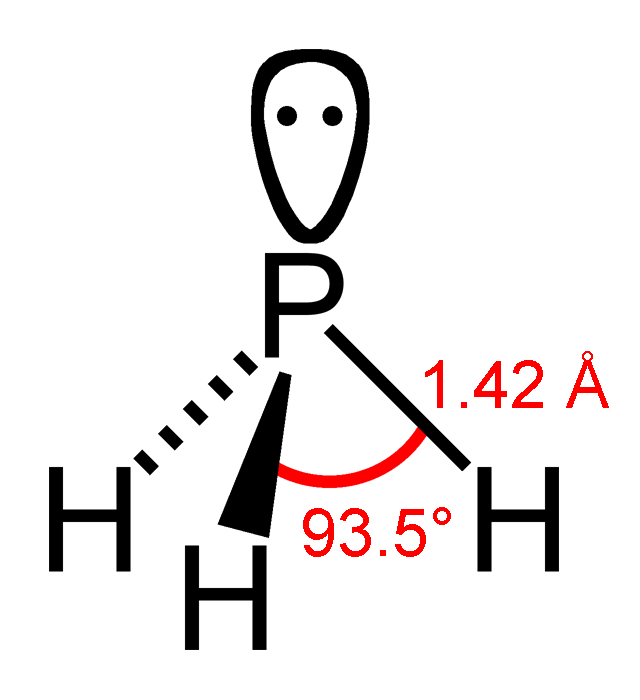
Pnictogen Hydride
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, bismuth, and moscovium) covalently bonded to hydrogen. Pnictogen trihydrides The simplest series has the chemical formula XH3 (less commonly H3X), with X representing any of the pnictogens. They take on the pyramidal structure (as opposed to the trigonal planar arrangement of the group 13 hydrides), and therefore are polar. These pnictogen trihydrides are generally increasingly unstable and poisonous with heavier elements. Some properties of the pnictogen trihydrides follow: These gases have no smell in pure form, instead gaining it when in contact with air. Ammonia has an infamous, intense odour resembling urine and/or fish, commonly the result of the decomposition of urea. Phosphine smells like fish or garlic, and stibine like rotten eggs, similar to hydrogen sulfide a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binary Compounds Of Hydrogen
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types. Overview Binary hydrogen compounds in group 1 are the ionic hydrides (also called saline hydrides) wherein hydrogen is bound electrostatically. Because hydrogen is located somewhat centrally in an electronegative sense, it is necessary for the counterion to be exceptionally electropositive for the hydride to possibly be accurately described as truly behaving ionic. Therefore, this category of hydrides contains only a few members. Hydrides in group 2 are polymeric covalent hydrides. In these, hydrogen forms bridging covalent bonds, usually possessing mediocre degrees of ionic character, which make them difficult to be accurately described as either covalent or ionic. The one exception is beryl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |