|
8-Cyclopentyl-1,3-dimethylxanthine
8-Cyclopentyl-1,3-dimethylxanthine (8-Cyclopentyltheophylline, 8-CPT, CPX) is a drug which acts as a potent and selective antagonist for the adenosine receptors, with some selectivity for the A1 receptor subtype, as well as a non-selective phosphodiesterase inhibitor. It has stimulant effects in animals with slightly higher potency than caffeine. See also * 8-Chlorotheophylline * 8-Phenyltheophylline * DMPX * DPCPX * Xanthine Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine ... References Adenosine receptor antagonists Phosphodiesterase inhibitors Xanthines Cyclopentyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Receptor
The adenosine receptors (or P1 receptors) are a class of purinergic G protein-coupled receptors with adenosine as the endogenous ligand. There are four known types of adenosine receptors in humans: A1, A2A, A2B and A3; each is encoded by a different gene. The adenosine receptors are commonly known for their antagonists caffeine and theophylline, whose action on the receptors produces the stimulating effects of coffee, tea and chocolate. Pharmacology Each type of adenosine receptor has different functions, although with some overlap. For instance, both A1 receptors and A2A play roles in the heart, regulating myocardial oxygen consumption and coronary blood flow, while the A2A receptor also has broader anti-inflammatory effects throughout the body. These two receptors also have important roles in the brain, regulating the release of other neurotransmitters such as dopamine and glutamate, while the A2B and A3 receptors are located mainly peripherally and are involved in proce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caffeine
Caffeine is a central nervous system (CNS) stimulant of the methylxanthine class. It is mainly used recreationally as a cognitive enhancer, increasing alertness and attentional performance. Caffeine acts by blocking binding of adenosine to the adenosine A1 receptor, which enhances release of the neurotransmitter acetylcholine. Caffeine has a three-dimensional structure similar to that of adenosine, which allows it to bind and block its receptors. Caffeine also increases cyclic AMP levels through nonselective inhibition of phosphodiesterase. Caffeine is a bitter, white crystalline purine, a methylxanthine alkaloid, and is chemically related to the adenine and guanine bases of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). It is found in the seeds, fruits, nuts, or leaves of a number of plants native to Africa, East Asia and South America, and helps to protect them against herbivores and from competition by preventing the germination of nearby seeds, as well as en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine A1 Receptor
The adenosine A1 receptor is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand. Biochemistry A1 receptors are implicated in sleep promotion by inhibiting wake-promoting cholinergic neurons in the basal forebrain. A1 receptors are also present in smooth muscle throughout the vascular system. The adenosine A1 receptor has been found to be ubiquitous throughout the entire body. Signalling Activation of the adenosine A1 receptor by an agonist causes binding of Gi1/2/3 or Go protein. Binding of Gi1/2/3 causes an inhibition of adenylate cyclase and, therefore, a decrease in the cAMP concentration. An increase of the inositol triphosphate/diacylglycerol concentration is caused by an activation of phospholipase C, whereas the elevated levels of arachidonic acid are mediated by DAG lipase, which cleaves DAG to form arachidonic acid. Several types of potassium channels are activated but N-, P-, and Q-type calcium channels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DPCPX
8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, PD-116,948) is a drug which acts as a potent and selective antagonist for the adenosine A1 receptor. It has high selectivity for A1 over other adenosine receptor subtypes, but as with other xanthine derivatives DPCPX also acts as a phosphodiesterase inhibitor, and is almost as potent as rolipram at inhibiting PDE4. It has been used to study the function of the adenosine A1 receptor in animals, which has been found to be involved in several important functions such as regulation of breathing and activity in various regions of the brain, and DPCPX has also been shown to produce behavioural effects such as increasing the hallucinogen-appropriate responding produced by the 5-HT2A agonist DOI, and the dopamine release induced by MDMA, as well as having interactions with a range of anticonvulsant drugs. See also * DMPX * CPX * Xanthine Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a puri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DMPX
DMPX (3,7-dimethyl-1-propargylxanthine) is a caffeine analog which displays affinity to A2 adenosine receptors, in contrast to the A1 subtype receptors. DMPX had 28× and 15× higher potency than caffeine in blocking peripheral and central NECA-responses. The locomotor stimulation caused by DMPX (ED50 10 ''μ''mol/kg) was similarly higher than caffeine. See also * DPCPX * 8-PT * CPX (8-CPT) * 8-Chlorotheophylline * Theophylline Theophylline, also known as 1,3-dimethylxanthine, is a phosphodiesterase inhibiting drug used in therapy for respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma under a variety of brand names. As a member of the ... References {{Adenosinergics Adenosine receptor antagonists Anxiogenics Caffeine Designer drugs Vasoconstrictors Propargyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
8-Phenyltheophylline
8-Phenyltheophylline (8-phenyl-1,3-dimethylxanthine, 8-PT) is a drug derived from the xanthine family which acts as a potent and selective antagonist for the adenosine receptors A1 and A2A, but unlike other xanthine derivatives has virtually no activity as a phosphodiesterase inhibitor. It has stimulant effects in animals with similar potency to caffeine. Coincidentally 8-phenyltheophylline has also been found to be a potent and selective inhibitor of the liver enzyme CYP1A2 which makes it likely to cause interactions with other drugs which are normally metabolised by CYP1A2. See also * 8-Chlorotheophylline * 8-Cyclopentyltheophylline * DPCPX * DMPX * Xanthine Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine ... References Adenosine receptor antagonists Xanthi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphodiesterase Inhibitors
A phosphodiesterase inhibitor is a drug that blocks one or more of the five subtypes of the enzyme phosphodiesterase (PDE), thereby preventing the inactivation of the intracellular second messengers, cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) by the respective PDE subtype(s). The ubiquitous presence of this enzyme means that non-specific inhibitors have a wide range of actions, the actions in the heart, and lungs being some of the first to find a therapeutic use. History The different forms or subtypes of phosphodiesterase were initially isolated from rat brains in the early 1970s and were soon afterward shown to be selectively inhibited in the brain and in other tissues by a variety of drugs. The potential for selective phosphodiesterase inhibitors as therapeutic agents was predicted as early as 1977 by Weiss and Hait. This prediction meanwhile has proved to be true in a variety of fields. Classification Nonselective PDE inhibitors Methyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Receptor Antagonists
Adenosine ( symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9-glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias. Adenosyl (abbreviated Ado or 5'-dAdo) is the chemical group formed by removal of the 5′-hydroxy (OH) group. It is found in adenosylcobalamin (an active form of vitamin B12) and as a radical in radical SAM enzymes. Medical uses Supraventricular tachycardia In individuals with supraventricular tachycardia (SVT), adenosine is used to help identify and convert the rh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine
Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine. Xanthine is a product on the pathway of purine degradation. * It is created from guanine by guanine deaminase. * It is created from hypoxanthine by xanthine oxidoreductase. * It is also created from xanthosine by purine nucleoside phosphorylase. Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme. Use and manufacturing Xanthine is used as a drug precursor for human and animal medications, and is manufactured as a pesticide ingredient. Clinical significance Derivatives of xanthine (known collectively as xanthines) are a group of alkaloids commonly used for their effects as mild stimulants and as bronchodilators, notably in the treatment of asthma or infl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalation, injection, smoking, ingestion, absorption via a patch on the skin, suppository, or dissolution under the tongue. In pharmacology, a drug is a chemical substance, typically of known structure, which, when administered to a living organism, produces a biological effect. A pharmaceutical drug, also called a medication or medicine, is a chemical substance used to treat, cure, prevent, or diagnose a disease or to promote well-being. Traditionally drugs were obtained through extraction from medicinal plants, but more recently also by organic synthesis. Pharmaceutical drugs may be used for a limited duration, or on a regular basis for chronic disorders. Pharmaceutical drugs are often classified into drug classes—groups of re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
8-Chlorotheophylline
8-Chlorotheophylline, also known as 1,3-dimethyl-8-chloroxanthine, is a stimulant drug of the xanthine chemical class, with physiological effects similar to caffeine. Its main use is in combination (salt) with diphenhydramine in the antiemetic dimenhydrinate (Dramamine). Diphenhydramine reduces nausea but causes drowsiness, and the stimulant properties of 8-Chlorotheophylline help reduce that side effect. Despite being classified as a xanthine stimulant, 8-chlorotheophylline can generally not produce any locomotor activity above control in mice and does not appear to cross the blood-brain barrier well. The 8-chloro modification is not selected for pharmacological properties; instead, it was to raise the acidity of the xanthine amine group enough to form a co-salt with diphenhydramine.10.1126/science.109.2840.574.a The drug is also sold in combination with promethazine Promethazine is a first-generation antihistamine, antipsychotic, sedative, and antiemetic used to treat al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
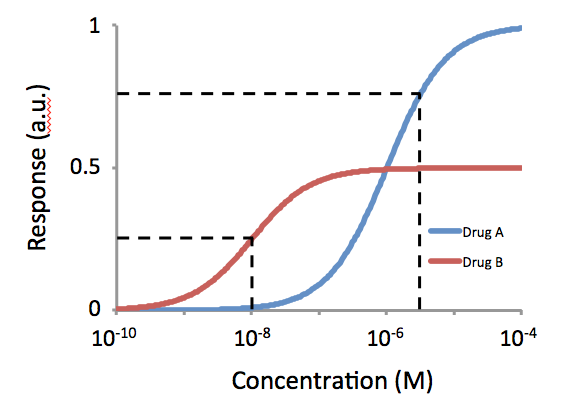
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency ( meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |