|
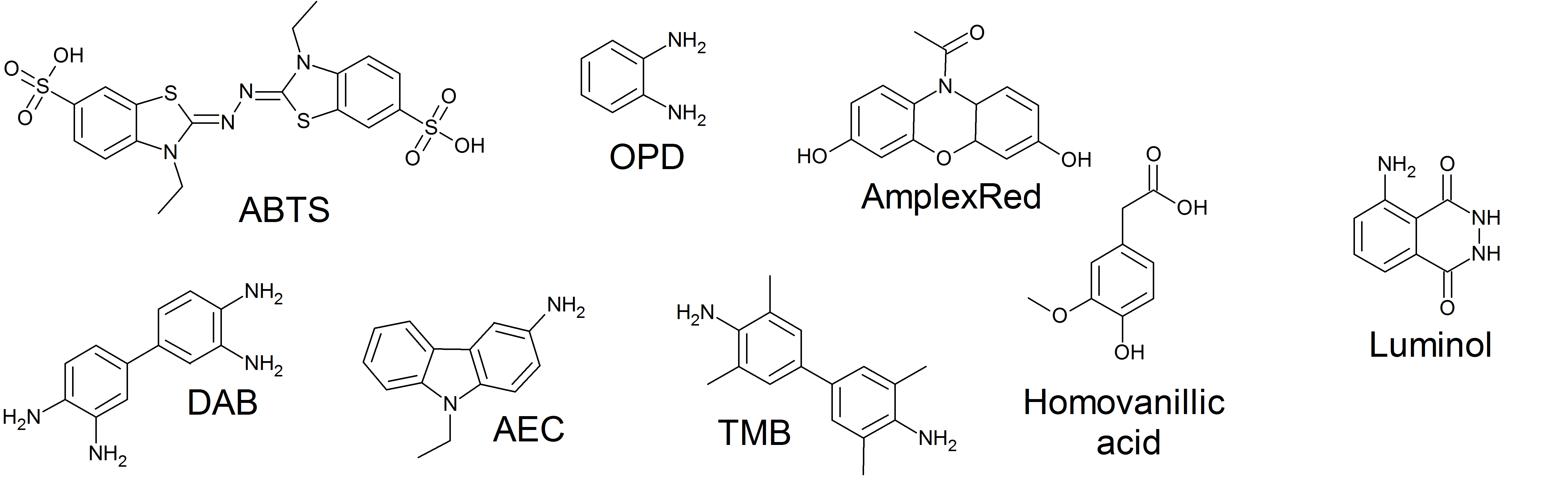
3,3’,5,5’-tetramethylbenzidine
3,3′,5,5′-Tetramethylbenzidine or TMB is a chromogenic substrate used in staining procedures in immunohistochemistry as well as being a visualising reagent used in enzyme-linked immunosorbent assays (ELISA). TMB is a white solid that forms a pale blue-green liquid in solution with ethyl acetate. TMB is degraded by sunlight and by fluorescent lights. Used to detect hematuria as it turns blue in contact with hemoglobin. Enzymatic assay TMB can act as a hydrogen donor for the reduction of hydrogen peroxide to water by peroxidase enzymes such as horseradish peroxidase. The resulting one-electron oxidation product is a diimine-diamine complex, which causes the solution to take on a blue colour, and this colour change can be read on a spectrophotometer at the wavelengths of 370 and 650 nm. The reaction can be halted by addition of acid or another stop reagent. Using sulfuric acid turns TMB yellow, with a Absorption (electromagnetic radiation), peak absorbance of 450 nm. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ELISA
The enzyme-linked immunosorbent assay (ELISA) (, ) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand (commonly an amino acid) in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a medical diagnosis, diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries. In the most simple form of an ELISA, antigens from the sample to be tested are attached to a surface. Then, a matching antibody is applied over the surface so it can bind the antigen. This antibody is linked to an enzyme, and then any unbound antibodies are removed. In the final step, a substance containing the enzyme's Enzyme substrate, substrate is added. If there was binding, the subsequent reaction produces a detectable signal, most commonly a color change. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
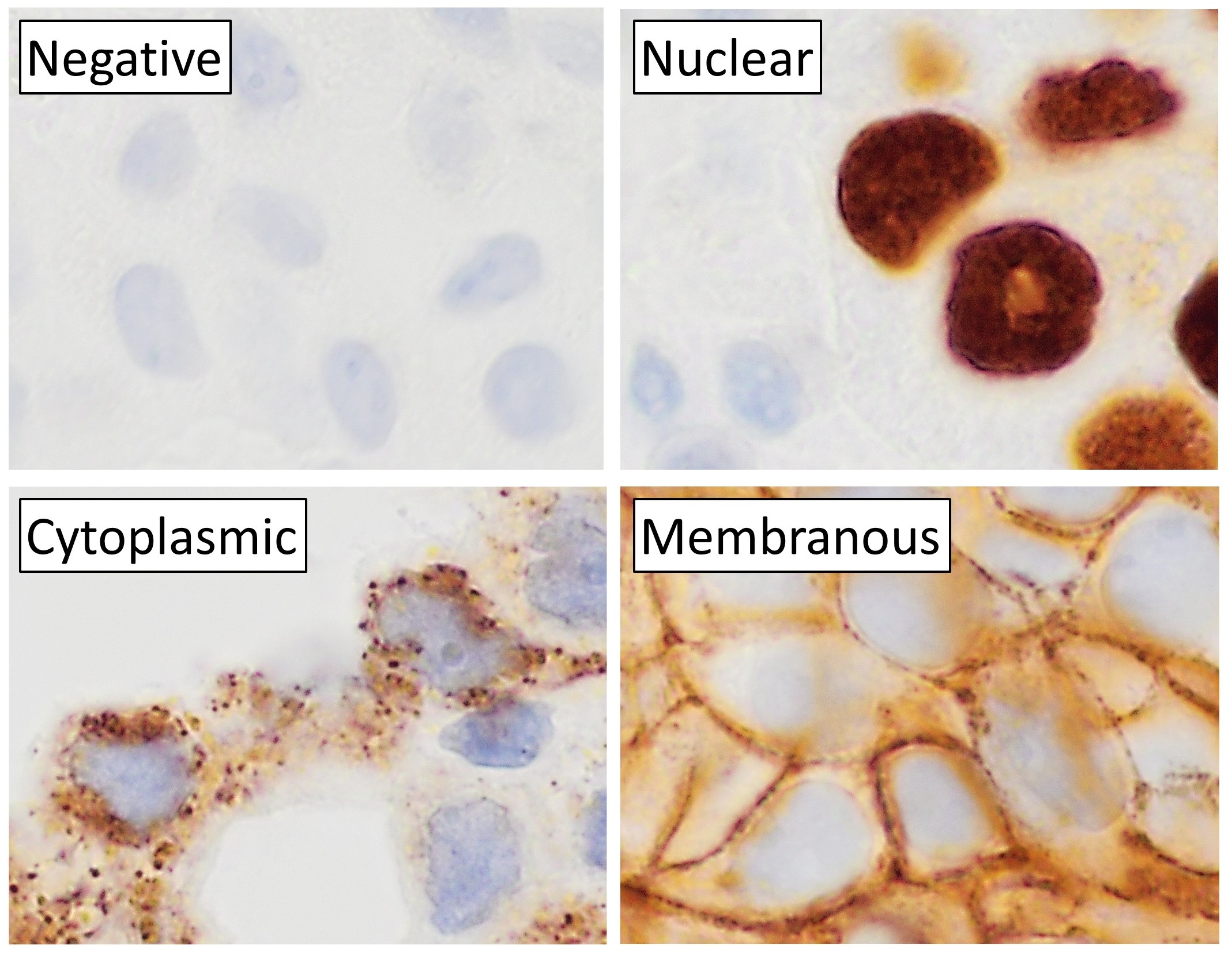
Immunohistochemistry
Immunohistochemistry is a form of immunostaining. It involves the process of selectively identifying antigens in cells and tissue, by exploiting the principle of Antibody, antibodies binding specifically to antigens in biological tissues. Albert Coons, Albert Hewett Coons, Ernst Berliner, Ernest Berliner, Norman Jones and Hugh J Creech was the first to develop immunofluorescence in 1941. This led to the later development of immunohistochemistry. Immunohistochemical staining is widely used in the diagnosis of abnormal cells such as those found in cancerous tumors. In some cancer cells certain tumor antigens are expressed which make it possible to detect. Immunohistochemistry is also widely used in basic research, to understand the distribution and localization of biomarkers and differentially expressed proteins in different parts of a biological tissue. Sample preparation Immunohistochemistry can be performed on tissue that has been fixed and embedded in Paraffin wax, paraffin, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diimine
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diimines and 1,3-diimines. These compounds are used as ligands, but they are also precursors to other organic compounds. Preparation Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated. Many are derived from the condensation of 1,2-diketones and dialdehydes with amines, often anilines. The dialdehyde glyoxal is an especially common precursor. Similar methods are used to prepare Schiff bases and oximes. 1,2-Diimines The 1,2-diimines are also called α-diimines and 1,4-diazabutadienes. An example is glyoxal-bis(mesitylimine), a yellow solid that is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal. 2,2'-Bipyridine is a 1,2-diimine. 1,2-Diketimines are “ non-innocent ligands”, akin to the dithiolenes. : 1,3-Diimines For example, acetylacetone (2,4-pentanedione) and a primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzidine
Benzidine (trivial name), also called 1,1'-biphenyl-4,4'-diamine (systematic name), is an organic compound with the chemical formula, formula (C6H4NH2)2. It is an aromatic amine. It is a component of a test for cyanide. Related derivatives are used in the organic synthesis, production of dyes. Benzidine has been linked to bladder cancer, bladder and pancreatic cancer. Synthesis and properties Benzidine is prepared in a two step process from nitrobenzene. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a rearrangement reaction to 4,4'-benzidine. Smaller amounts of other isomers are also formed. The benzidine rearrangement, which proceeds intramolecularly, is a classic reaction mechanism, mechanistic puzzle in organic chemistry. : The conversion is described as a [5,5] sigmatropic reaction. : In terms of its physical properties, 4,4'-benzidine is poorly sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ames Test
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a bioassay, biological assay to assess the mutagenic potential of chemical compounds. A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer is often linked to mutation. The test serves as a quick and convenient assay to estimate the carcinogenic potential of a compound because standard carcinogen assays on mice and rats are time-consuming (taking two to three years to complete) and expensive. However, false-positives and false-negatives are known. The procedure was described in a series of papers in the early 1970s by Bruce Ames and his group at the University of California, Berkeley. General procedure The Ames test uses several strains of the bacterium ''Salmonella typhimurium'' that carry mutations in genes involved in histidine synthesis. These strains a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (electromagnetic Radiation)
In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy—and so transforms electromagnetic energy into internal energy of the absorber (for example, thermal energy). A notable effect of the absorption of electromagnetic radiation is attenuation of the radiation; attenuation is the gradual reduction of the intensity of light waves as they propagate through a medium. Although the absorption of waves does not usually depend on their intensity (linear absorption), in certain conditions (optics) the medium's transparency changes by a factor that varies as a function of wave intensity, and saturable absorption (or nonlinear absorption) occurs. Quantifying absorption Many approaches can potentially quantify radiation absorption, with key examples following. * The absorption coefficient along with some closely related derived quantities * The attenuation coefficient (NB used infrequently with meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, and hydrogen, with the molecular formula . It is a colorless, odorless, and Viscosity, viscous liquid that is Miscibility, miscible with water. Pure sulfuric acid does not occur naturally due to its Dehydration reaction, strong affinity to water vapor; it is Hygroscopy, hygroscopic and readily absorbs water vapor from the Atmosphere of Earth, air. Concentrated sulfuric acid is a strong oxidant with powerful dehydrating properties, making it highly corrosive towards other materials, from rocks to metals. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid but, to the contrary, dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Horseradish Peroxidase
The enzyme horseradish peroxidase (HRP), found in the roots of horseradish, is used extensively in biochemistry applications. It is a metalloenzyme with many isoforms, of which the most studied type is C. It catalyzes the oxidation of various organic substrates by hydrogen peroxide. Structure The structure of the enzyme was first solved by X-ray crystallography in 1997; and has since been solved several times with various substrates. It is a large alpha-helix, alpha-helical glycoprotein which binds heme as a redox Cofactor (biochemistry), cofactor. Substrates Alone, the HRP enzyme, or conjugates thereof, is of little value; its presence must be made visible using a Substrate (biochemistry), substrate that, when Redox, oxidized by HRP using hydrogen peroxide as the oxidizing agent, yields a characteristic color change that is detectable by spectrophotometric methods. Numerous substrates for horseradish peroxidase have been described and commercialized to exploit the desir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |