|
Benzidine
Benzidine (trivial name), also called 1,1'-biphenyl-4,4'-diamine (systematic name), is an organic compound with the chemical formula, formula (C6H4NH2)2. It is an aromatic amine. It is a component of a test for cyanide. Related derivatives are used in the organic synthesis, production of dyes. Benzidine has been linked to bladder cancer, bladder and pancreatic cancer. Synthesis and properties Benzidine is prepared in a two step process from nitrobenzene. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a rearrangement reaction to 4,4'-benzidine. Smaller amounts of other isomers are also formed. The benzidine rearrangement, which proceeds intramolecularly, is a classic reaction mechanism, mechanistic puzzle in organic chemistry. : The conversion is described as a [5,5] sigmatropic reaction. : In terms of its physical properties, 4,4'-benzidine is poorly sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzidine Rearrangement
Benzidine ( trivial name), also called 1,1'- biphenyl-4,4'-diamine (systematic name), is an organic compound with the formula (C6H4NH2)2. It is an aromatic amine. It is a component of a test for cyanide. Related derivatives are used in the production of dyes. Benzidine has been linked to bladder and pancreatic cancer. Synthesis and properties Benzidine is prepared in a two step process from nitrobenzene. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a rearrangement reaction to 4,4'-benzidine. Smaller amounts of other isomers are also formed. The benzidine rearrangement, which proceeds intramolecularly, is a classic mechanistic puzzle in organic chemistry. : The conversion is described as a ,5 sigmatropic reaction. : In terms of its physical properties, 4,4'-benzidine is poorly soluble in cold water but can be recrystallized from hot water, where it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,3'-Dichlorobenzidine
3,3'-Dichlorobenzidine is an organic compound with the formula (C6H3Cl(NH2))2. The pure compound is pale yellow, but commercial samples are often colored. It is barely soluble in water and is often supplied as a wet paste. It is widely used in the production of diarylide yellow pigments used in the production of printing inks. Its use in the production of dyes has been largely discontinued because of concerns about carcinogenicity. Preparation and reactions 3,3'-Dichlorobenzidine is prepared in two steps from 2-nitrochlorobenzene. The first step involves reduction with zinc in base to afford 2,2'-dichlorodiphenylhydrazine. This intermediate undergoes the benzidine rearrangement to afford 3,3'-dichlorobenzidine. Aqueous solutions of 3,3'-dichlorobenzidine degrade in light to monochloro derivative. It undergoes chlorination (for example in water treatment plants) to give the tetrachloro derivative. The most widely practiced reaction of 3,3'-dichlorobenzidine is its double diazoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-Dianisidine
''o''-Dianisidine is an organic compound with the formula CH3O)(H2N)C6H3sub>2. A colorless or white solid, it is a bifunctional compound derived via the benzidine rearrangement from ''o''-anisidine. ''o''-Dianisidine is a precursor to some azo dyes by formation of the bis(diazonium) derivative, which is coupled to diverse aromatic compounds. Some commercial dyes derived from ''o''-dianisidine include C. I. Direct Blue 1, 15, 22, 84, and 98.. ''o''-Dianisidine is also used in assaying activity of peroxidase in lab. The general reaction of a peroxidase is as follows. :ROOR' + \overset + 2H+ -> ce + R'OH Where the ROOR' can be hydrogen peroxide, and the electron donor be ''o''-dianisidine. Safety The manufacture and degradation of ''o''-dianisidine, like other benzidene derivatives, has attracted regulatory attention. It is also used as a reagent in biochemistry in testing for peroxide In chemistry, peroxides are a group of Chemical compound, compounds with the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tolidine
2-Tolidine (orthotolidine, o-tolidine; not to be confused with ''o''-toluidine) is an organic compound with the chemical formula . Several isomers are known; the 3-tolidine derivative is also important commercially. It is a colorless compound although commercial samples are often colored. It is slightly soluble in water. It forms salts with acids, such as the hydrochloride, which is commercially available. 2-Tolidine can be produced by benzidine rearrangement from a hydrazone derivative of 2-nitrotoluene. : Uses 2-Tolidine is an aromatic amine used mainly for dye production.K. Hunger. W. Herbst "Pigments, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. 2-Tolidine is an intermediate for the production of soluble azo dyes and insoluble pigments used particularly in the textile, leather and paper industries. It is also used for the production of certain elastomers. left, 200px, Orthotolidine in chlorine test kit 2-Tolidine was widely u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
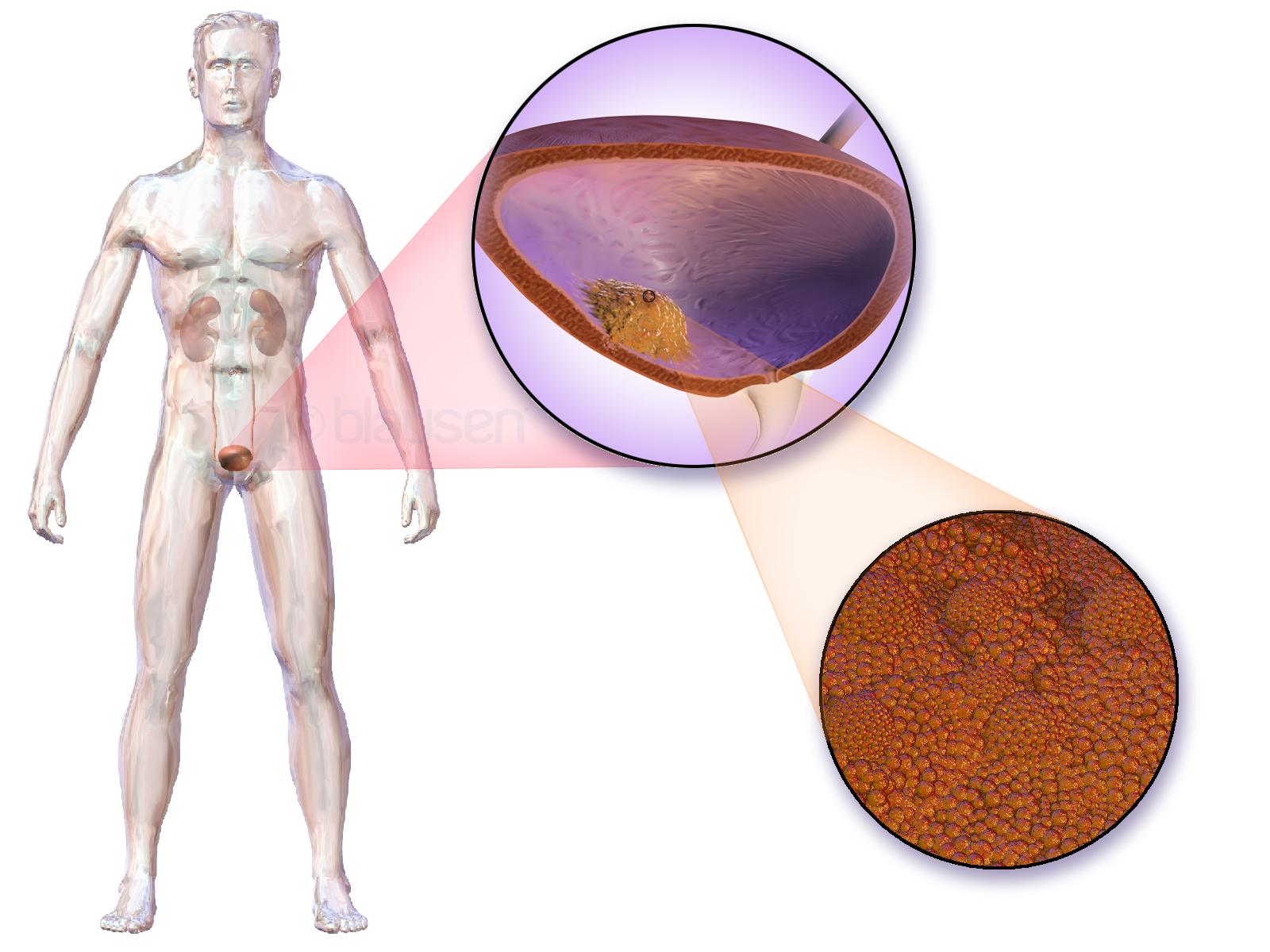
Bladder Cancer
Bladder cancer is the abnormal growth of cells in the bladder. These cells can grow to form a tumor, which eventually spreads, damaging the bladder and other organs. Most people with bladder cancer are diagnosed after noticing blood in their urine. Those suspected of having bladder cancer typically have their bladder inspected by a thin medical camera, a procedure called cystoscopy. Suspected tumors are removed and examined to determine if they are cancerous. Based on how far the tumor has spread, the cancer case is assigned a stage 0 to 4; a higher stage indicates a more widespread and dangerous disease. Those whose bladder tumors have not spread outside the bladder have the best prognoses. These tumors are typically surgically removed, and the person is treated with chemotherapy or one of several immune-stimulating therapies. Those whose tumors continue to grow, or whose tumors have penetrated the bladder muscle, often have their bladder surgically removed ( radical cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one hydrogen (the site at which it is attached) may use the prefixes xenyl or diphenylyl. It has a distinctively pleasant smell. Biphenyl is an aromatic hydrocarbon with a molecular formula (C6H5)2. It is notable as a starting material for the production of polychlorinated biphenyls (PCBs), which were once widely used as dielectric fluids and heat transfer agents. Biphenyl is also an intermediate for the production of a host of other organic compounds such as emulsifiers, optical brighteners, crop protection products, and plastics. Biphenyl is insoluble in water, but soluble in typical organic solvents. The biphenyl molecule consists of two connected phenyl rings. Properties and occurrence Biphenyl is a solid at room temperature, wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Aminonaphthalene-4-sulfonic Acid
Aminonaphthalenesulfonic acids are compounds with the composition C10H6(NH2)(SO3H), being derived from naphthalene (C10H8) substituted by an amino and sulfonic acid groups. These compounds are colorless solids. They are useful precursors to dyes.Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. . Notes: Peri-acid dehydrates to the sultam. Via the Bucherer reaction, heating periacid with anilinium salts gives the N-phenyl derivative, precursor to Acid Blue 113. {, class="wikitable" style="margin-left: auto; margin-right: auto; border: none;" , + 2-Aminonaphthalenesulfonic acids , - ! scope="col" , Isomer ! scope="col" , CAS Registry Number ! scope="col" , Alternative names ! scope="col" , Preparative route and notes , - , 2-Aminonaphthalene-1-sulfonic acid, , 81-16-3, , Tobias acid, , Bucherer reaction The Bucherer reaction in organic chemistry is the reversible conversion of a naphthol to a naphthyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematic Name
A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivial name is a name that has at least one systematic part and at least one trivial name, trivial part, such as a chemical common name, vernacular name. Creating systematic names can be as simple as assigning a prefix or a number to each object (in which case they are a type of numbering scheme), or as complex as encoding the complete structure of the object in the name. Many systems combine some information about the named object with an extra sequence number to make it into a unique identifier. Systematic names often co-exist with earlier common names assigned before the creation of any systematic naming system. For example, many common chemicals are still referred to by their common or trivial names, even by chemists. In chemistry In ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Luminol
Luminol (C8H7N3O2) is a chemical that exhibits chemiluminescence, with a blue glow, when mixed with an appropriate oxidizing agent. Luminol is a white-to-pale-yellow Crystal, crystalline solid that is soluble in most polar organic solvents but insoluble in water. Forensic investigators use luminol to detect trace amounts of blood at crime scenes, as it reacts with the iron in hemoglobin. Biologists use it in cellular assays to detect copper, iron, and cyanides as well as specific proteins via western blotting. When luminol is sprayed evenly across an area, trace amounts of an activating oxidant make the luminol emit a blue glow that can be seen in a darkened room. The glow only lasts about 30 seconds but can be documented photographically. The glow is stronger in areas receiving more spray; the intensity of the glow does not indicate the amount of blood or other activator present. Synthesis Luminol is synthesized in a two-step process, beginning with 3-nitrophthalic acid. Fir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |