|
1,1,2-Trifluoroethane
1,1,2-Trifluoroethane or R-143, is a hydrofluorocarbon with formula . It is a colourless gas at room temperature. It is an asymmetrical isomer of 1,1,1-trifluoroethane. 1,1,2-Trifluoroethane has a global warming potential of 397 for 100 years.G. Myhre, D. Shindell et al.: Climate Change 2013: The Physical Science Basis. Working Group I contribution to the IPCC Fifth Assessment Report. Hrsg.: Intergovernmental Panel on Climate Change. 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, 24–39; Table 8.SM.16 1,1,2-Trifluoroethane can be obtained by the hydrogenation of 1,2-dichlorodifluoroethylene or chlorotrifluoroethylene. See also * 1,2-Dichloro-1,1,2-trifluoroethane * 1,1,2-Trichloro-1,2,2-trifluoroethane * 1,1,2-Trichloroethane 1,1,2-Trichloroethane, vinyl trichloride or 1,1,2-TCA, is an organochloride solvent with the molecular formula and the structural formula . It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroethylene
Trifluoroethylene (abbreviated as TrFE) is an organofluoride compound with the chemical formula . It is a colourless gas.Lide, D.R. CRC Handbook of Chemistry and Physics 88TH Edition 2007-2008. CRC Press, Taylor & Francis, Boca Raton, FL 2007, p. 3-500 TrFE can polymerise to form poly(trifluoroethylene) (PTrFE). It can also form copolymer In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are som ...s with other monomers, such as vinylidene fluoride to form a co-polymer that is used to produce ferroelectric materials. References {{reflist Monomers Fluoroalkenes Hydrofluoroolefins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-trifluoroethane
1,1,1-Trifluoroethane, or R-143a or simply trifluoroethane, is a hydrofluorocarbon (HFC) compound that is a colorless gas. It should not be confused with the much more commonly used HFC gas R-134a, nor confused with the isomeric compound 1,1,2-trifluoroethane. 1,1,1-Trifluoroethane has a critical temperature of 73 °C. Applications Trifluoroethane is used as a refrigerant either by itself or more commonly as a component of blended mixtures. It is also used as a propellant in canned air products used to clean electronic equipment. Environmental effects Unlike CFCs used as refrigerants, trifluoroethane has no chlorine atoms and therefore is not ozone-depleting. Its high chemical stability and infra-red absorbency make it a potent greenhouse gas with a lifetime of about 50 years and a global warming potential of 4300, which are at the high end compared to many other commonly used HFC refrigerants. Its abundance in the atmosphere more than doubled from about 10 p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,2-Trichloroethane
1,1,2-Trichloroethane, vinyl trichloride or 1,1,2-TCA, is an organochloride solvent with the molecular formula and the structural formula . It is a colourless, sweet-smelling liquid that does not dissolve in water, but is soluble in most organic solvents. It is an isomer of 1,1,1-trichloroethane, and a byproduct of its manufacture. It is used as a solvent and as an intermediate in the synthesis of 1,1-dichloroethylene. Toxicity 1,1,2-Trichloroethane may be harmful by inhalation, ingestion, and skin contact. It is a respiratory and eye irritant. 1,1,2-TCA is a central nervous system depressant and inhalation of vapors may cause dizziness, drowsiness, headache, nausea, shortness of breath, and unconsciousness. The Occupational Safety and Health Administration and National Institute for Occupational Safety and Health The National Institute for Occupational Safety and Health (NIOSH, ) is the List of United States federal agencies, United States federal agency responsible f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluorocarbon
Hydrofluorocarbons (HFCs) are synthetic organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs) such as R-12, which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) such as R-21 which are presently being phased out. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection. HFCs may not harm the ozone layer as much as the compounds they replace, but they still contribute to global warming – with some like trifluoromethane (CHF3 or R-23) having 11,700 times the warming potential of carbon dioxide. HFC atmo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Global Warming Potential
Global warming potential (GWP) is a measure of how much heat a greenhouse gas traps in the atmosphere over a specific time period, relative to carbon dioxide (). It is expressed as a multiple of warming caused by the same mass of carbon dioxide (). Therefore, by definition has a GWP of 1. For other gases it depends on how strongly the gas absorbs thermal radiation, how quickly the gas leaves the atmosphere, and the time frame considered. For example, Methane emissions, methane has a GWP over 20 years (GWP-20) of 81.2. meaning that, a Fugitive gas emissions, leak of a tonne of methane is equivalent to emitting 81.2 tonnes of carbon dioxide measured over 20 years. As methane has a much shorter atmospheric lifetime than carbon dioxide, its GWP is much less over longer time periods, with a GWP-100 of 27.9 and a GWP-500 of 7.95. The carbon dioxide equivalent (e or eq or -e or -eq) can be calculated from the GWP. For any gas, it is the mass of that would warm the earth as much as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-dichlorodifluoroethylene
A dichlorodifluoroethylene (systematically named dichlorodifluoroethene) is one of three compounds with the chemical formula . Dichlorodifluoroethylenes are colourless gases, and are some of the simplest chlorodifluoroalkenes. The structural isomers are used as intermediates or precursors in the production of other industrial chemicals. 1,1-Dichloro-2,2-difluoroethylene 1,1-Dichloro-2,2-difluoroethylene is a low-boiling liquid that is used a refrigerant. It may also be used as a solvent, but has practical limitations as such, because of its low boiling point (commercial listings, 19 °C; lit. 17 °C). It is regarded as a hazardous chemical for being toxic by inhalation (see MSDS), and a low-boiling liquid, and it causes irritation when it comes into contact with the skin and mucous membranes. Its ASHRAE The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE ) is an American professional association seeking to advance heating, ve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorotrifluoroethylene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota. Production and reactions Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc: :CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2 In 2012, an estimated 1–10 million pounds were produced commercially in the United States. Addition of iodine monochloride to chlorotrifluoroethylene gives iododichlorotrifluoroethane: : The latter is a precursor to hexafluorobutadiene. Thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Dichloro-1,1,2-trifluoroethane
1,2-Dichloro-1,1,2-trifluoroethane is a volatile liquid chlorofluoroalkane composed of carbon, hydrogen, chlorine and fluorine, and with structural formula CClF2CHClF. It is also known as a refrigerant with the designation R-123a. Formation 1,1,2-Trichloro-1,2,2-trifluoroethane can be biotransformed in sewage sludge to 1,2-dichloro-1,1,2-trifluoroethane. Properties The critical temperature Critical or Critically may refer to: *Critical, or critical but stable, medical states **Critical, or intensive care medicine *Critical juncture, a discontinuous change studied in the social sciences. *Critical Software, a company specializing in ... of R-123a is . The rotation of the molecule appears to be hindered by the present of chlorine on each carbon atom, but is eased at higher temperatures. Use Although not deliberately used, R-123a is a significant impurity in its isomer, the widely used 2,2-dichloro-1,1,1-trifluoroethane (R-123). References External links * {{DEFAULTSORT:Dic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
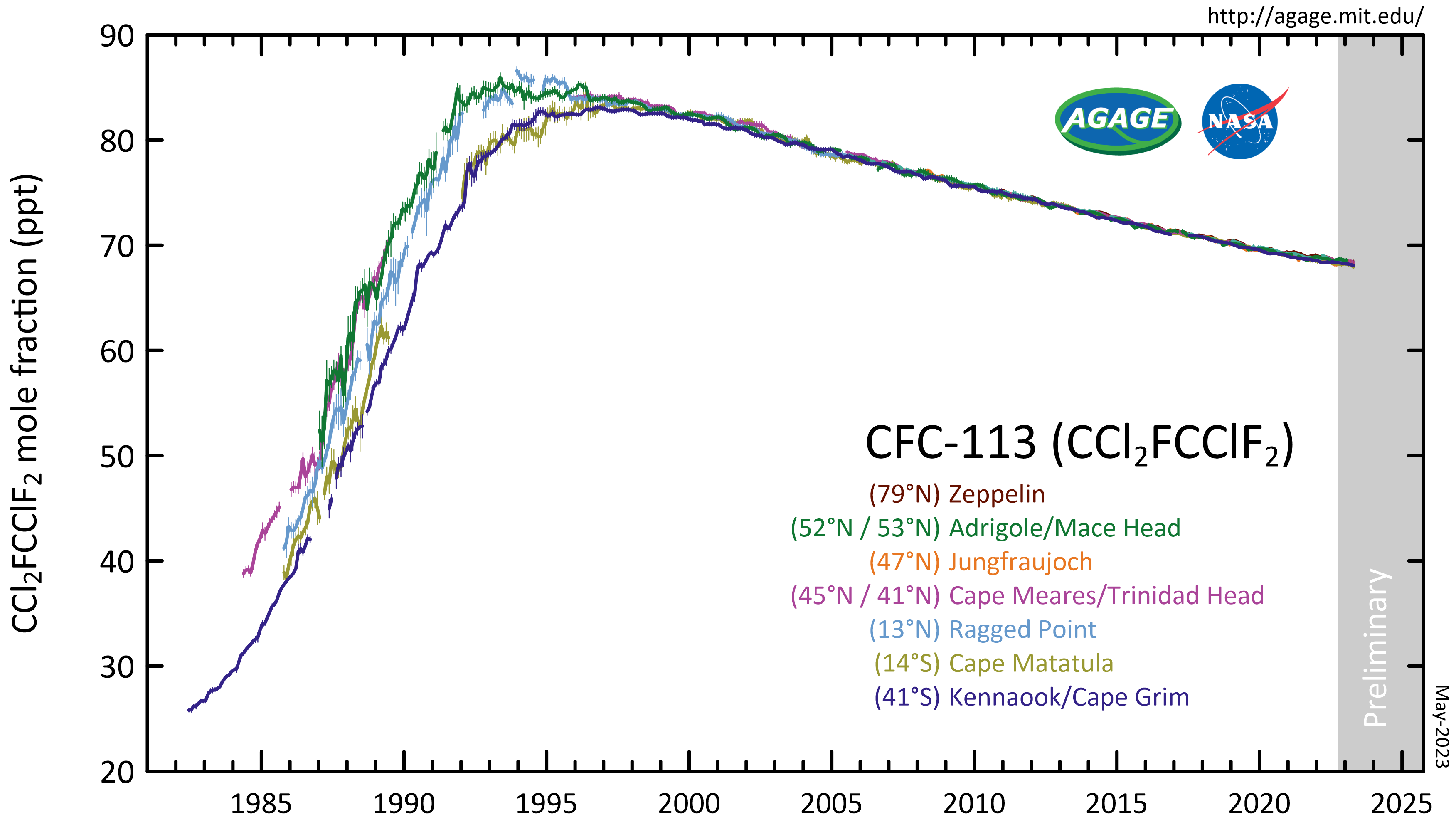
1,1,2-Trichloro-1,2,2-trifluoroethane
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane (often abbreviated as TCTFE) or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Production CFC-113 can be prepared from hexachloroethane and hydrofluoric acid: : This reaction may require catalysts such as antimony, chromium, iron and alumina at high temperatures. Another synthesis method uses HF on tetrachloroethylene instead. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It may remain in the atmosphere up to 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (UV, sunlight in the 190-225 nm range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrofluorocarbons
Hydrofluorocarbons (HFCs) are synthetic organic compounds that contain fluorine and hydrogen atoms, and are the most common type of organofluorine compounds. Most are gases at room temperature and pressure. They are frequently used in air conditioning and as refrigerants; R-134a (1,1,1,2-tetrafluoroethane) is one of the most commonly used HFC refrigerants. In order to aid the recovery of the stratospheric ozone layer, HFCs were adopted to replace the more potent chlorofluorocarbons (CFCs) such as R-12, which were phased out from use by the Montreal Protocol, and hydrochlorofluorocarbons (HCFCs) such as R-21 which are presently being phased out. HFCs are also used in insulating foams, aerosol propellants, as solvents and for fire protection. HFCs may not harm the ozone layer as much as the compounds they replace, but they still contribute to global warming – with some like trifluoromethane (CHF3 or R-23) having 11,700 times the warming potential of carbon dioxide. HFC atmo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |