hydrolysis on:
[Wikipedia]
[Google]
[Amazon]
 Hydrolysis (; ) is any chemical reaction in which a molecule of
Hydrolysis (; ) is any chemical reaction in which a molecule of
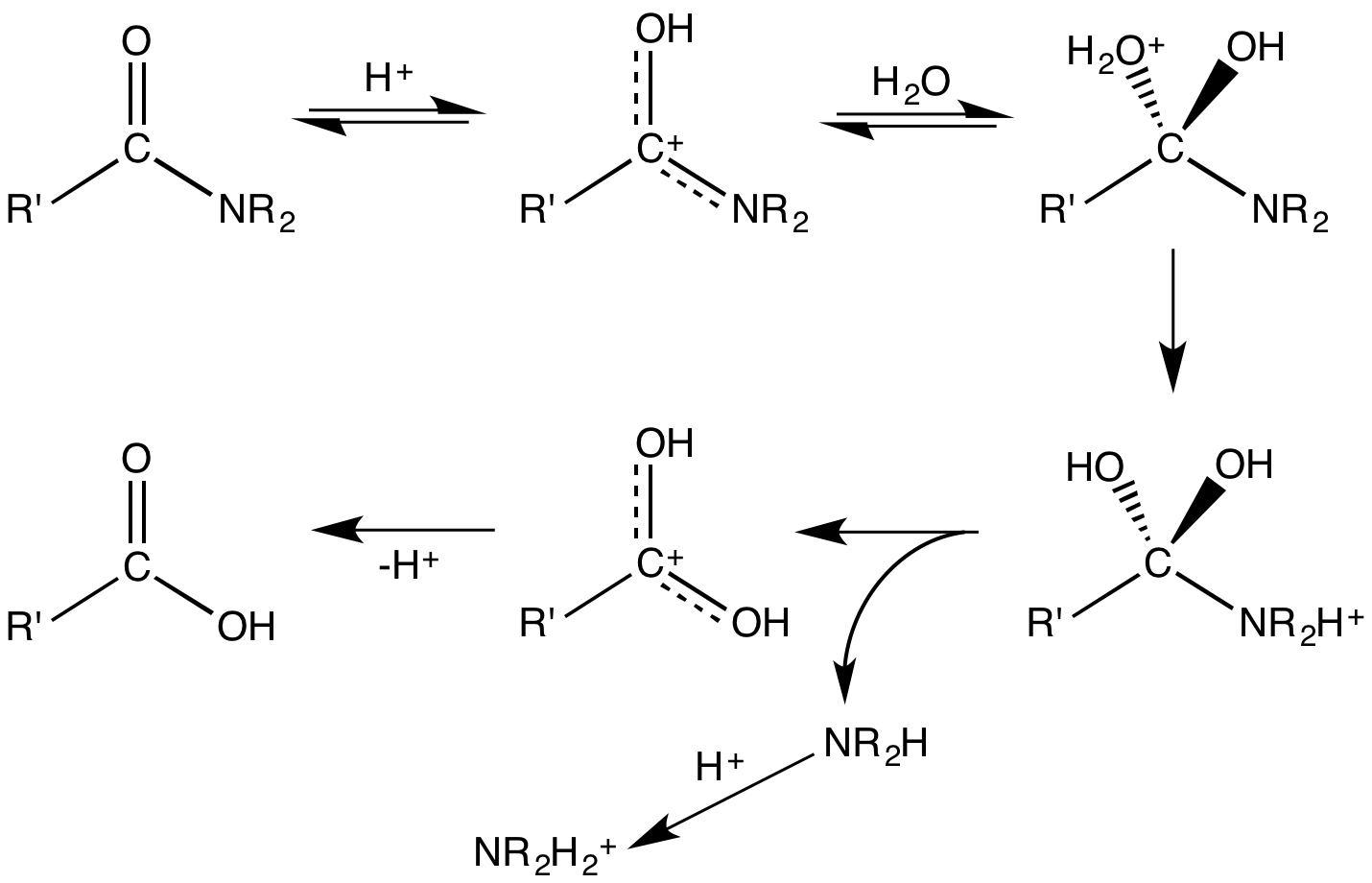 Upon hydrolysis, an amide converts into a
Upon hydrolysis, an amide converts into a
 Monosaccharides can be linked together by glycosidic bonds, which can be cleaved by hydrolysis. Two, three, several or many monosaccharides thus linked form disaccharides, trisaccharides, oligosaccharides, or
Monosaccharides can be linked together by glycosidic bonds, which can be cleaved by hydrolysis. Two, three, several or many monosaccharides thus linked form disaccharides, trisaccharides, oligosaccharides, or
 The reaction is often used to solubilize solid organic matter. Chemical drain cleaners take advantage of this method to dissolve hair and fat in pipes. The reaction is also used to dispose of human and other animal remains as an alternative to traditional burial or cremation.
The reaction is often used to solubilize solid organic matter. Chemical drain cleaners take advantage of this method to dissolve hair and fat in pipes. The reaction is also used to dispose of human and other animal remains as an alternative to traditional burial or cremation.
 Hydrolysis (; ) is any chemical reaction in which a molecule of
Hydrolysis (; ) is any chemical reaction in which a molecule of water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
and fructose
Fructose (), or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and gal ...
), this is recognized as saccharification.
Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water.
Types
Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and water molecule to split into two parts. In such reactions, one fragment of the target molecule (or parent molecule) gains ahydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particl ...
. It breaks a chemical bond in the compound.
Salts
A common kind of hydrolysis occurs when asalt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
of a weak acid or weak base (or both) is dissolved in water. Water spontaneously ionizes into hydroxide anions and hydronium cations. The salt also dissociates into its constituent anions and cations. For example, sodium acetate dissociates in water into sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
and acetate ions. Sodium ions react very little with the hydroxide ions whereas the acetate ions combine with hydronium ions to produce acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
. In this case the net result is a relative excess of hydroxide ions, yielding a basic solution.
Strong acids also undergo hydrolysis. For example, dissolving sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
() in water is accompanied by hydrolysis to give hydronium and bisulfate, the sulfuric acid's conjugate base. For a more technical discussion of what occurs during such a hydrolysis, see Brønsted–Lowry acid–base theory.
Esters and amides
Acid–base-catalysed hydrolyses are very common; one example is the hydrolysis of amides orester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s. Their hydrolysis occurs when the nucleophile (a nucleus-seeking agent, e.g., water or hydroxyl ion) attacks the carbon of the carbonyl group of the ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
or amide. In an aqueous base, hydroxyl ions are better nucleophiles than polar molecules such as water. In acids, the carbonyl group becomes protonated, and this leads to a much easier nucleophilic attack. The products for both hydrolyses are compounds with carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
groups.
Perhaps the oldest commercially practiced example of ester hydrolysis is saponification (formation of soap). It is the hydrolysis of a triglyceride
A triglyceride (from '' tri-'' and '' glyceride''; also TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids.
Triglycerides are the main constituents of body fat in humans and other vertebrates ...
(fat) with an aqueous base such as sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
(NaOH). During the process, glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pha ...
is formed, and the fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s react with the base, converting them to salts. These salts are called soaps, commonly used in households.
In addition, in living systems, most biochemical reactions (including ATP hydrolysis) take place during the catalysis of enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s. The catalytic action of enzymes allows the hydrolysis of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s, fats, oils, and carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
s. As an example, one may consider proteases (enzymes that aid digestion by causing hydrolysis of peptide bonds in protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s). They catalyze the hydrolysis of interior peptide bonds in peptide chains, as opposed to exopeptidases (another class of enzymes, that catalyze the hydrolysis of terminal peptide bonds, liberating one free amino acid at a time).
However, proteases do not catalyze the hydrolysis of all kinds of proteins. Their action is stereo-selective: Only proteins with a certain tertiary structure are targeted as some kind of orienting force is needed to place the amide group in the proper position for catalysis. The necessary contacts between an enzyme and its substrates (proteins) are created because the enzyme folds in such a way as to form a crevice into which the substrate fits; the crevice also contains the catalytic groups. Therefore, proteins that do not fit into the crevice will not undergo hydrolysis. This specificity preserves the integrity of other proteins such as hormone
A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physio ...
s, and therefore the biological system continues to function normally.
 Upon hydrolysis, an amide converts into a
Upon hydrolysis, an amide converts into a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
and an amine or ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
(which in the presence of acid are immediately converted to ammonium salts). One of the two oxygen groups on the carboxylic acid are derived from a water molecule and the amine (or ammonia) gains the hydrogen ion. The hydrolysis of peptides
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Dalton (unit), Da or more are called proteins. Chains of fewer t ...
gives amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s.
Many polyamide polymers such as nylon 6,6 hydrolyze in the presence of strong acids. The process leads to depolymerization. For this reason nylon products fail by fracturing when exposed to small amounts of acidic water. Polyesters are also susceptible to similar polymer degradation reactions. The problem is known as environmental stress cracking.
ATP
Hydrolysis is related to energy metabolism and storage. All living cells require a continual supply of energy for two main purposes: thebiosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthe ...
of micro and macromolecules, and the active transport of ions and molecules across cell membranes. The energy derived from the oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of nutrients is not used directly but, by means of a complex and long sequence of reactions, it is channeled into a special energy-storage molecule, adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known ...
(ATP). The ATP molecule contains pyrophosphate linkages (bonds formed when two phosphate units are combined) that release energy when needed. ATP can undergo hydrolysis in two ways: Firstly, the removal of terminal phosphate to form adenosine diphosphate
Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbon ...
(ADP) and inorganic phosphate, with the reaction:
:
Secondly, the removal of a terminal diphosphate to yield adenosine monophosphate (AMP) and pyrophosphate. The latter usually undergoes further cleavage into its two constituent phosphates. This results in biosynthesis reactions, which usually occur in chains, that can be driven in the direction of synthesis when the phosphate bonds have undergone hydrolysis.
Polysaccharides
polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
s, respectively. Enzymes that hydrolyze glycosidic bonds are called " glycoside hydrolases" or "glycosidases".
The best-known disaccharide is sucrose (table sugar). Hydrolysis of sucrose yields glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
and fructose
Fructose (), or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and gal ...
. Invertase is a sucrase used industrially for the hydrolysis of sucrose to so-called invert sugar. Lactase is essential for digestive hydrolysis of lactose in milk; many adult humans do not produce lactase and cannot digest the lactose in milk.
The hydrolysis of polysaccharides to soluble sugars can be recognized as saccharification. Malt made from barley
Barley (), a member of the grass family, is a major cereal grain grown in temperate climates globally. It was one of the first cultivated grains; it was domesticated in the Fertile Crescent around 9000 BC, giving it nonshattering spikele ...
is used as a source of β-amylase to break down starch into the disaccharide maltose, which can be used by yeast to produce beer. Other amylase
An amylase () is an enzyme that catalysis, catalyses the hydrolysis of starch (Latin ') into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large ...
enzymes may convert starch to glucose or to oligosaccharides. Cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
is first hydrolyzed to cellobiose by cellulase and then cellobiose is further hydrolyzed to glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
by beta-glucosidase. Ruminants such as cows are able to hydrolyze cellulose into cellobiose and then glucose because of symbiotic bacteria that produce cellulases.
DNA
Hydrolysis of DNA occurs at a significant rate in vivo.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993 Apr 22;362(6422):709-15. doi: 10.1038/362709a0. PMID 8469282 For example, it is estimated that in each human cell 2,000 to 10,000 DNA purine bases turn over every day due to hydrolytic depurination, and that this is largely counteracted by specific rapid DNA repair processes. Hydrolytic DNA damages that fail to be accurately repaired may contribute tocarcinogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cell (biology), cells are malignant transformation, transformed into cancer cells. The process is characterized by changes at the cellular, G ...
and ageing
Ageing (or aging in American English) is the process of becoming older until death. The term refers mainly to humans, many other animals, and fungi; whereas for example, bacteria, perennial plants and some simple animals are potentially biol ...
.
Metal aqua ions
Metal ions are Lewis acids, and in aqueous solution they form metal aquo complexes of the general formula . The aqua ions undergo hydrolysis, to a greater or lesser extent. The first hydrolysis step is given generically as : Thus the aqua cations behave as acids in terms of Brønsted–Lowry acid–base theory. This effect is easily explained by considering the inductive effect of the positively charged metal ion, which weakens the bond of an attached water molecule, making the liberation of a proton relatively easy. The dissociation constant, pKa, for this reaction is more or less linearly related to the charge-to-size ratio of the metal ion. Ions with low charges, such as are very weak acids with almost imperceptible hydrolysis. Large divalent ions such as , , and have a pKa of 6 or more and would not normally be classed as acids, but small divalent ions such as undergo extensive hydrolysis. Trivalent ions like and are weak acids whose pKa is comparable to that ofacetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
. Solutions of salts such as or in water are noticeably acidic; the hydrolysis can be suppressed by adding an acid such as nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
, making the solution more acidic.
Hydrolysis may proceed beyond the first step, often with the formation of polynuclear species via the process of olation. Some "exotic" species such as are well characterized. Hydrolysis tends to proceed as pH rises leading, in many cases, to the precipitation of a hydroxide such as or . These substances, major constituents of bauxite
Bauxite () is a sedimentary rock with a relatively high aluminium content. It is the world's main source of aluminium and gallium. Bauxite consists mostly of the aluminium minerals gibbsite (), boehmite (γ-AlO(OH)), and diaspore (α-AlO(OH) ...
, are known as laterites and are formed by leaching from rocks of most of the ions other than aluminium and iron and subsequent hydrolysis of the remaining aluminium and iron.
Mechanism strategies
Acetal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments n ...
s, imines, and enamines can be converted back into ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s by treatment with excess water under acid-catalyzed conditions: ; ; .
Catalysis
Acidic hydrolysis
Acid catalysis can be applied to hydrolyses. For example, in the conversion ofcellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
or starch to glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
. Carboxylic acids can be produced from acid hydrolysis of esters.
Acids catalyze hydrolysis of nitriles to amides. Acid hydrolysis ''does not'' usually refer to the acid catalyzed addition of the elements of water to double or triple bonds by electrophilic addition as may originate from a hydration reaction. Acid hydrolysis is used to prepare monosaccharide with the help of mineral acids but formic acid and trifluoroacetic acid have been used.
Acid hydrolysis can be utilized in the pretreatment of cellulosic material, so as to cut the interchain linkages in hemicellulose and cellulose.
Alkaline hydrolysis
Alkaline hydrolysis usually refers to types of nucleophilic substitution reactions in which the attacking nucleophile is a hydroxide ion. The best known type is saponification: cleavingester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s into carboxylate salts and alcohols. In ester hydrolysis, the hydroxide ion nucleophile attacks the carbonyl carbon. This mechanism is supported by isotope labeling experiments. For example, when ethyl propionate with an oxygen-18 labeled ethoxy group is treated with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
(NaOH), the oxygen-18 is completely absent from the sodium propionate product and is found exclusively in the ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
formed.
: The reaction is often used to solubilize solid organic matter. Chemical drain cleaners take advantage of this method to dissolve hair and fat in pipes. The reaction is also used to dispose of human and other animal remains as an alternative to traditional burial or cremation.
The reaction is often used to solubilize solid organic matter. Chemical drain cleaners take advantage of this method to dissolve hair and fat in pipes. The reaction is also used to dispose of human and other animal remains as an alternative to traditional burial or cremation.
See also
*Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cell (biology), cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known ...
* Water cremation
* Catabolism
* Condensation reaction
* Dehydration reaction
* Hydrolysis constant
* Inhibitor protein
* Polymer degradation
* Proteolysis
* Saponification
* Sol–gel polymerisation
* Solvolysis
In chemistry, solvolysis is a type of nucleophilic substitution (S1/S2) or elimination reaction, elimination where the nucleophile is a solvent molecule. Characteristic of S1 reactions, solvolysis of a chirality (chemistry), chiral reactant affor ...
* Thermal hydrolysis
References
{{Authority control Chemical reactions Reactions of esters Equilibrium chemistry