Quadruple bond on:
[Wikipedia]
[Google]
[Amazon]
A quadruple bond is a type of 
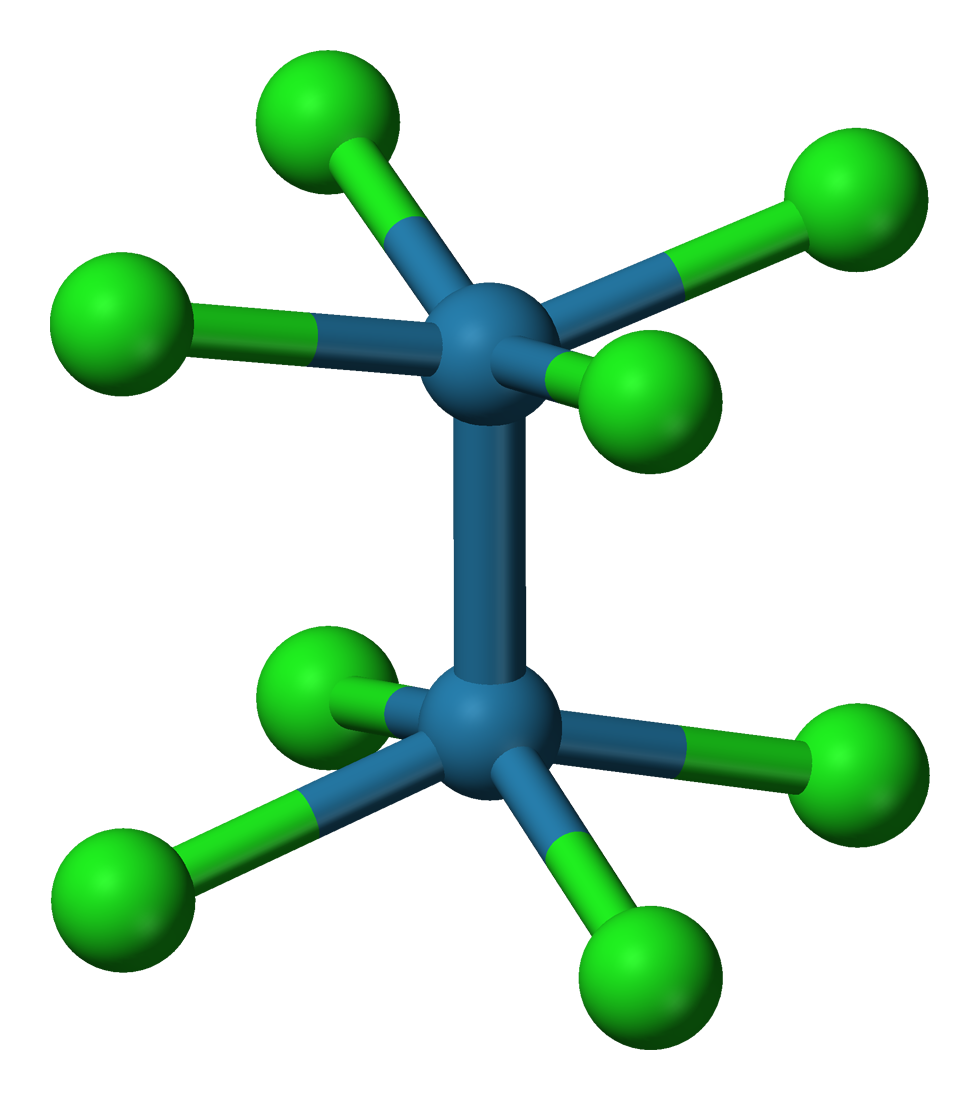
 The e2Cl8sup>2−
The e2Cl8sup>2−
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
between two atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
s involving eight electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s. This bond is an extension of the more familiar types double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
s and triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
s. Stable quadruple bonds are most common among the transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s in the middle of the , such as rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
, tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isolat ...
, technetium
Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous ...
, molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
and chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
. Typically the ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s that support quadruple bonds are π-donors, not π-acceptors.
History
Chromium(II) acetate
Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the formula Cr2(CH3CO2)4(H2O)2. This formula is commonly abbreviated Cr2(OAc)4(H2O)2. This red-coloured compound features a quadruple bond. The prepara ...
, Cr2(''μ''-O2CCH3)4(H2O)2, was the first chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
containing a quadruple bond to be synthesized. It was described in 1844 by E. Peligot, although its distinctive bonding was not recognized for more than a century.
The first crystallographic study of a compound with a quadruple bond was provided by Soviet chemists for salts of . The very short Re–Re distance was noted. This short distance (and the salt's diamagnetism) indicated Re–Re bonding. These researchers however misformulated the anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
as a derivative of Re(II), i.e., .
Soon thereafter, F. Albert Cotton
Frank Albert Cotton FRS (April 9, 1930 – February 20, 2007) was an American chemist. He was the W.T. Doherty-Welch Foundation Chair and Distinguished Professor of Chemistry at Texas A&M University. He authored over 1600 scientific articles. C ...
and C.B. Harris reported the crystal structure of potassium octachlorodirhenate
Potassium octachlorodirhenate(III) is an inorganic compound with the formula Potassium, K2Rhenium, Re2Chlorine, Cl8. This dark blue salt (chemistry), salt is well known as an early example of a compound featuring quadruple bond between its metal c ...
or K2 e2Cl8�2H2O. This structural analysis indicated that the previous characterization was mistaken. Cotton and Harris formulated a molecular orbital rationale for the bonding that explicitly indicated a quadruple bond. The rhenium–rhenium bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
in this compound is only 224 pm. In molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
, the bonding is described as σ2π4δ2 with one sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
, two pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
s and one delta bond
In chemistry, delta bonds (δ bonds) are covalent chemical bonds, where four lobes of one involved atomic orbital overlap four lobes of the other involved atomic orbital. This overlap leads to the formation of a bonding molecular orbital with tw ...
.
Structure and bonding

 The e2Cl8sup>2−
The e2Cl8sup>2− ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
adopts an eclipsed conformation
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X–A–B–Y is 0°. Such a conformation can exist in any open chain, ...
as shown at left. The delta bonding orbital is then formed by overlap of the d orbitals on each rhenium atom, which are perpendicular to the Re–Re axis and lie in between the Re–Cl bonds. The d orbitals directed along the Re–Cl bonds are stabilized by interaction with chlorine ligand orbitals and do not contribute to Re–Re bonding. In contrast, the s2Cl8sup>2− ion with two more electrons (σ2π4δ2δ*2) has an Os–Os triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
and a staggered geometry.
Many other compounds with quadruple bonds between transition metal atoms have been described, often by Cotton and his coworkers. Isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the ...
with the dirhenium compound is the salt K4 o2Cl8(potassium octachlorodimolybdate
Potassium octachlorodimolybdate (systematically named potassium bis(tetrachloromolybdate)(''Mo''–''Mo'')(4−)) is an inorganic compound with the chemical formula . It is known as a red-coloured, microcrystalline solid. The anion is of historic ...
). An example of a ditungsten compound with a quadruple bond is ditungsten tetra(hpp)
Tetrakis(hexahydropyrimidinopyrimidine)ditungsten(II), known as ditungsten tetra(hpp), is the name of the coordination compound with the formula W2(hpp)4. This material consists of a pair of tungsten centers linked by the conjugate base of four ...
.
Quadruple bonds between atoms of main-group element
In chemistry and atomic physics, the main group is the group of elements (sometimes called the representative elements) whose lightest members are represented by helium, lithium, beryllium, boron, carbon, nitrogen, oxygen, and fluorine as arrange ...
s are unknown. Molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
shows that there are two sets of paired electrons in the sigma system (one bonding, one antibonding), and two sets of paired electrons in a degenerate π-bonding set of orbitals. This adds up to give a bond order of 2, meaning that there exists a double bond between the two carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
atoms in a dicarbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed throug ...
(C2) molecule. The molecular orbital diagram
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A f ...
of diatomic carbon would show that there are two pi bonds and no sigma bonds. However, a recent paper by S. Shaik et al. has suggested that a quadruple bond exists in diatomic carbon, but this is disputed.
See also
*Covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
* Bond order
References
Further reading
* {{Chemical bonding theory Chemical bonding