Potassium peroxymonosulfate on:
[Wikipedia]
[Google]
[Amazon]
Potassium peroxymonosulfate is widely used as an
 Oxone oxidizes sulfides to sulfoxides and then to
Oxone oxidizes sulfides to sulfoxides and then to 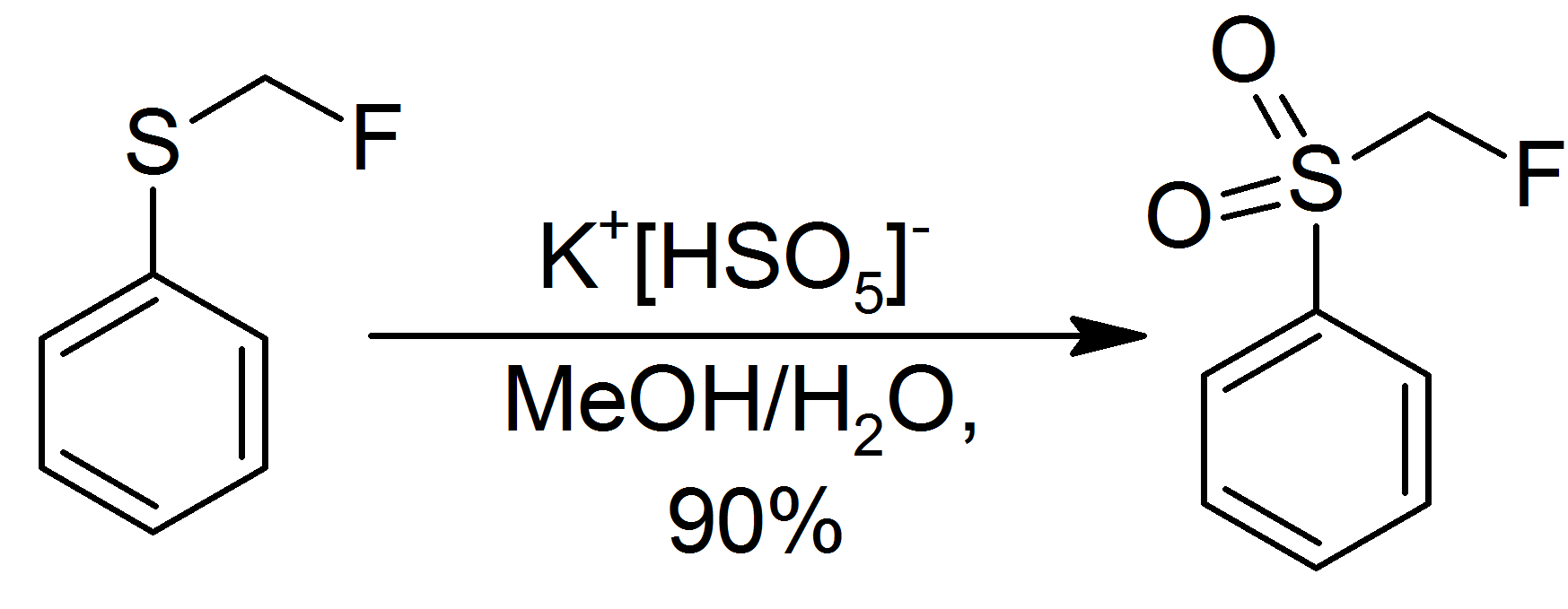 Oxone converts ketones to
Oxone converts ketones to 
oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
. It is the potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
of peroxymonosulfuric acid. Usually potassium peroxymonosulfate refers to the triple salt known as oxone.
The standard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as: ''"the value of the standard emf (electromotive force) of a cell in wh ...
for potassium peroxymonosulfate is +1.81 V with a half reaction
A half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction. ...
generating the hydrogen sulfate ():
::HSO5− + 2 H+ + 2 e− → HSO4− + H2O
Oxone
Potassium peroxymonosulfate per se is a relatively obscure salt, but its derivative called oxone is of commercial value. Oxone refers to the triple salt 2KHSO5·KHSO4·K2SO4. Oxone has a longer shelflife than does potassium peroxymonosulfate. A white, water-soluble solid, oxone loses <1% of its oxidizing power per month.Production
Oxone is produced from peroxysulfuric acid, which is generated in situ by combining oleum andhydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
. Careful neutralization of this solution with potassium hydroxide allows the crystallization of the triple salt.
Uses:lab use +chemistry research
Cleaning
Oxone is used widely for cleaning. It whitens dentures, disinfects swimming pools, and cleans chips for the manufacture of microelectronics.Organic chemistry
Oxone is a versatile oxidant in organic synthesis. It oxidizesaldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s to carboxylic acids; in the presence of alcoholic solvents, the ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s may be obtained. Internal alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s may be cleaved to two carboxylic acids (see below), while terminal alkenes may be epoxidized. Sulfides give sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s, tertiary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s give amine oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that contains the functional group , a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-grou ...
s, and phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s give phosphine oxides.
Further illustrative of the oxidative power of this salt is the conversion of an acridine
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups re ...
derivative to the corresponding acridine-N-oxide
In chemistry, an amine oxide, also known as an amine ''N''-oxide or simply ''N''-oxide, is a chemical compound that contains the functional group , a nitrogen-oxygen coordinate covalent bond with three additional hydrogen and/or substituent-grou ...
.
 Oxone oxidizes sulfides to sulfoxides and then to
Oxone oxidizes sulfides to sulfoxides and then to sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s.
 Oxone converts ketones to
Oxone converts ketones to dioxirane
In chemistry, dioxirane is a compound with formula , whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide.
Th ...
s. The synthesis of dimethyldioxirane
Dimethyldioxirane (DMDO), also referred to as Murray's reagent in reference to Robert W. Murray, is a dioxirane derived from acetone and can be considered as a monomer of acetone peroxide. It is a powerful yet selective oxidizing agent which fi ...
(DMDO) from acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
is representative. Dioxiranes are versatile oxidising agents and may be used for the epoxidation
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale ...
of olefins. In particular, if the starting ketone is chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
then the epoxide may be generated enantioselectively, which forms the basis of the Shi epoxidation
The Shi epoxidation is a chemical reaction described as the asymmetric epoxidation of alkenes with oxone (potassium peroxymonosulfate) and a fructose-derived catalyst (1). This reaction is thought to proceed via a dioxirane intermediate, generated ...
.

References
{{Reflist Persulfates Potassium compounds Oxidizing agents