Pelvic inflammatory disease on:
[Wikipedia]
[Google]
[Amazon]
Pelvic inflammatory disease, also known as pelvic inflammatory disorder (PID), is an
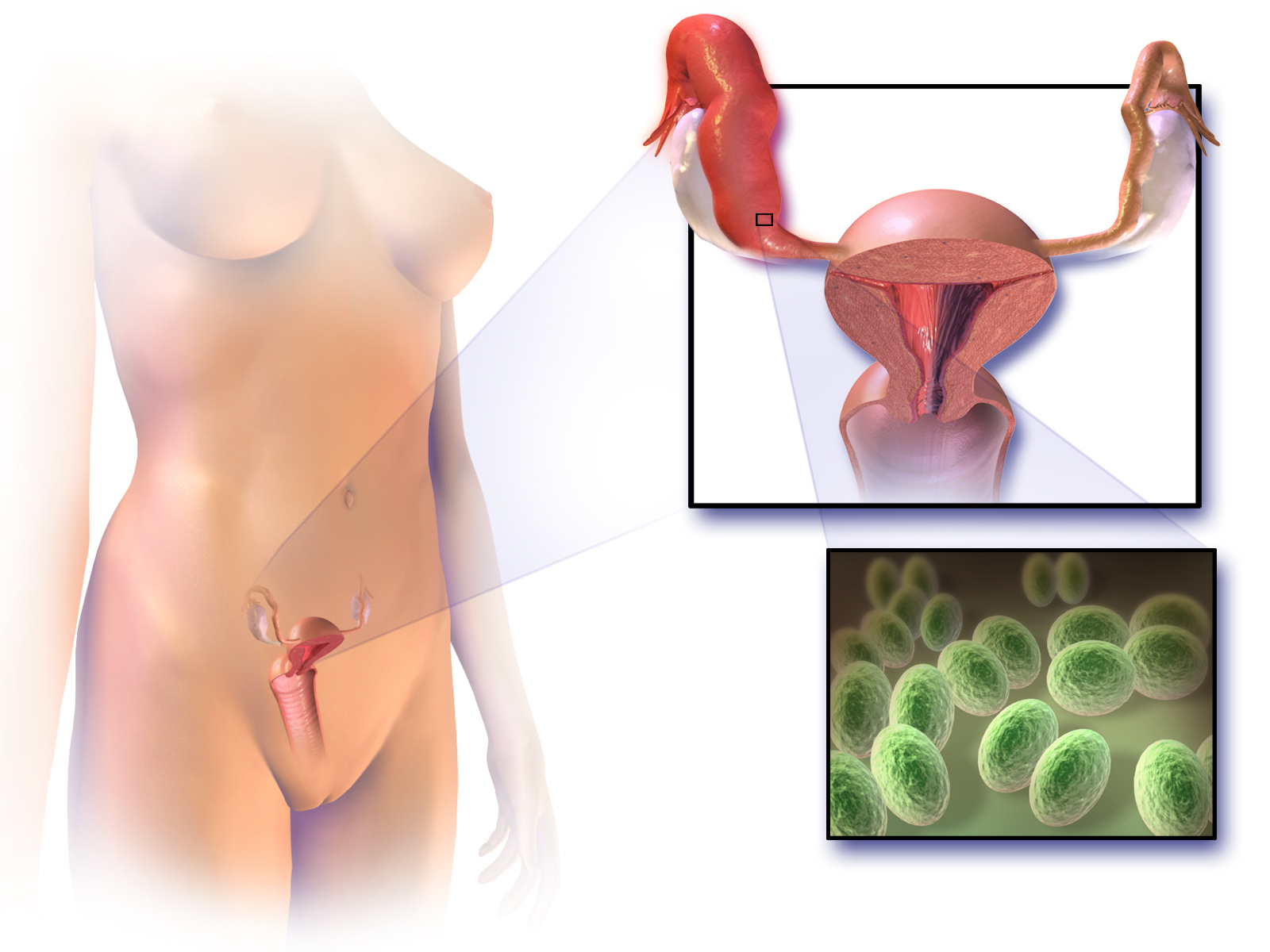 Symptoms in PID range from none to severe. If there are symptoms,
Symptoms in PID range from none to severe. If there are symptoms,

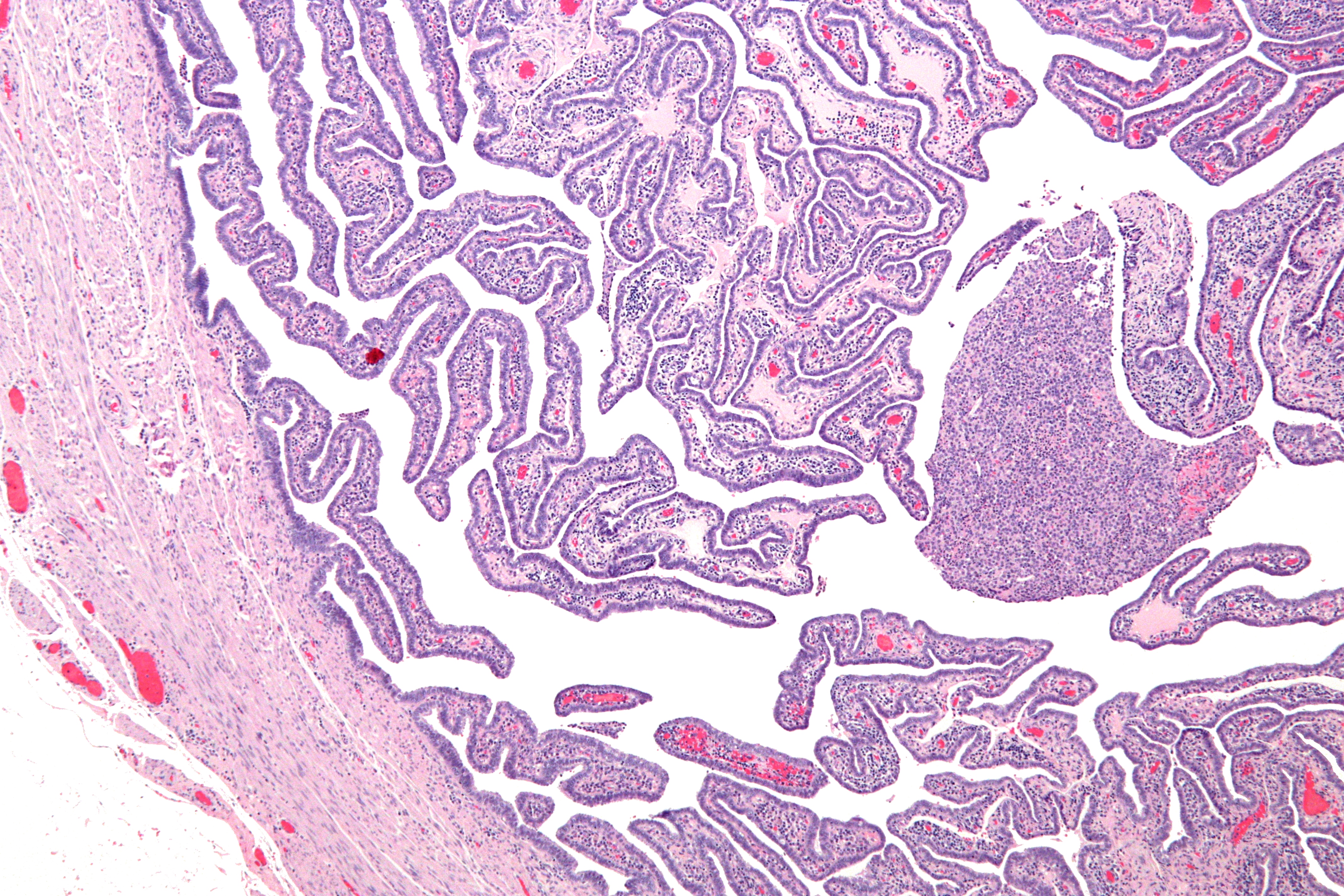 Upon a
Upon a
CDC
{{Authority control Abdominal pain Sexually transmitted diseases and infections Bacterial diseases Chlamydia infections Infections with a predominantly sexual mode of transmission Wikipedia medicine articles ready to translate Inflammatory diseases of female pelvic organs Mycoplasma Gonorrhea
infection
An infection is the invasion of tissues by pathogens, their multiplication, and the reaction of host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable dise ...
of the upper part of the female reproductive system
The female reproductive system is made up of the internal and external sex organs that function in the reproduction of new offspring. In humans, the female reproductive system is immature at birth and develops to maturity at puberty to be able ...
, namely the uterus
The uterus (from Latin ''uterus'', plural ''uteri'') or womb () is the organ in the reproductive system of most female mammals, including humans that accommodates the embryonic and fetal development of one or more embryos until birth. The uter ...
, fallopian tubes
The fallopian tubes, also known as uterine tubes, oviducts or salpinges (singular salpinx), are paired tubes in the human female that stretch from the uterus to the ovaries. The fallopian tubes are part of the female reproductive system. In ot ...
, and ovaries
The ovary is an organ in the female reproductive system that produces an ovum. When released, this travels down the fallopian tube into the uterus, where it may become fertilized by a sperm. There is an ovary () found on each side of the body. T ...
, and inside of the pelvis
The pelvis (plural pelves or pelvises) is the lower part of the trunk, between the abdomen and the thighs (sometimes also called pelvic region), together with its embedded skeleton (sometimes also called bony pelvis, or pelvic skeleton).
The ...
. Often, there may be no symptoms. Signs and symptoms, when present, may include lower abdominal pain, vaginal discharge
Vaginal discharge is a mixture of liquid, cells, and bacteria that lubricate and protect the vagina. This mixture is constantly produced by the cells of the vagina and cervix, and it exits the body through the vaginal opening. The composition, amou ...
, fever
Fever, also referred to as pyrexia, is defined as having a body temperature, temperature above the human body temperature, normal range due to an increase in the body's temperature Human body temperature#Fever, set point. There is not a single ...
, burning with urination
Dysuria refers to painful or uncomfortable urination.
It is one of a constellation of ''irritative'' bladder symptoms (also sometimes referred to as lower urinary tract symptoms), which includes nocturia and urinary frequency.
Diagnosis
The clin ...
, pain with sex
Dyspareunia ( ) is painful sexual intercourse due to medical or psychological causes. The term ''dyspareunia'' covers both female dyspareunia and male dyspareunia, but many discussions that use the term without further specification concern the ...
, bleeding after sex, or irregular menstruation
Irregular menstruation is a menstrual disorder whose manifestations include irregular cycle lengths as well as metrorrhagia (vaginal bleeding between expected periods). The possible causes of irregular menstruation may vary. The common factors of ...
. Untreated PID can result in long-term complications including infertility
Infertility is the inability of a person, animal or plant to reproduce by natural means. It is usually not the natural state of a healthy adult, except notably among certain eusocial species (mostly haplodiploid insects). It is the normal state ...
, ectopic pregnancy
Ectopic pregnancy is a complication of pregnancy in which the embryo attaches outside the uterus. Signs and symptoms classically include abdominal pain and vaginal bleeding, but fewer than 50 percent of affected women have both of these symptoms. ...
, chronic pelvic pain
Pelvic pain is pain in the area of the pelvis. Acute pain is more common than chronic pain. If the pain lasts for more than six months, it is deemed to be chronic pelvic pain. It can affect both the male and female pelvis.
Common causes in include ...
, and cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
.
The disease is caused by bacteria that spread from the vagina and cervix. Infections by ''Neisseria gonorrhoeae
''Neisseria gonorrhoeae'', also known as ''gonococcus'' (singular), or ''gonococci'' (plural), is a species of Gram-negative diplococci bacteria isolated by Albert Ludwig Sigesmund Neisser, Albert Neisser in 1879. It causes the sexually transmit ...
'' or ''Chlamydia trachomatis
''Chlamydia trachomatis'' (), commonly known as chlamydia, is a bacterium that causes chlamydia, which can manifest in various ways, including: trachoma, lymphogranuloma venereum, nongonococcal urethritis, cervicitis, salpingitis, pelvic inflamma ...
'' are present in 75 to 90 percent of cases. Often, multiple different bacteria are involved. Without treatment, about 10 percent of those with a chlamydial infection
Chlamydia, or more specifically a chlamydia infection, is a sexually transmitted infection caused by the bacterium ''Chlamydia trachomatis''. Most people who are infected have no symptoms. When symptoms do appear they may occur only several wee ...
and 40 percent of those with a gonorrhea infection
Gonorrhea, colloquially known as the clap, is a sexually transmitted infection (STI) caused by the bacterium ''Neisseria gonorrhoeae''. Infection may involve the genitals, mouth, or rectum. Infected men may experience pain or burning with ur ...
will develop PID. Risk factors are generally similar to those of sexually transmitted infection
Sexually transmitted infections (STIs), also referred to as sexually transmitted diseases (STDs) and the older term venereal diseases, are infections that are Transmission (medicine), spread by Human sexual activity, sexual activity, especi ...
s and include a high number of sexual partners and drug use. Vaginal douching may also increase the risk. The diagnosis is typically based on the presenting signs and symptoms. It is recommended that the disease be considered in all women of childbearing age who have lower abdominal pain. A definitive diagnosis of PID is made by finding pus
Pus is an exudate, typically white-yellow, yellow, or yellow-brown, formed at the site of inflammation during bacterial or fungal infection. An accumulation of pus in an enclosed tissue space is known as an abscess, whereas a visible collection ...
involving the fallopian tubes during surgery
Surgery ''cheirourgikē'' (composed of χείρ, "hand", and ἔργον, "work"), via la, chirurgiae, meaning "hand work". is a medical specialty that uses operative manual and instrumental techniques on a person to investigate or treat a pat ...
. Ultrasound
Ultrasound is sound waves with frequency, frequencies higher than the upper audible limit of human hearing range, hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hea ...
may also be useful in diagnosis.
Efforts to prevent the disease include not having sex or having few sexual partners and using condom
A condom is a sheath-shaped barrier device used during sexual intercourse to reduce the probability of pregnancy or a sexually transmitted infection (STI). There are both male and female condoms. With proper use—and use at every act of in ...
s. Screening women at risk for chlamydial infection followed by treatment decreases the risk of PID. If the diagnosis is suspected, treatment is typically advised. Treating a woman's sexual partners should also occur. In those with mild or moderate symptoms, a single injection of the antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
ceftriaxone
Ceftriaxone, sold under the brand name Rocephin, is a third-generation cephalosporin antibiotic used for the treatment of a number of bacterial infections. These include middle ear infections, endocarditis, meningitis, pneumonia, bone and joint ...
along with two weeks of doxycycline
Doxycycline is a broad-spectrum tetracycline class antibiotic used in the treatment of infections caused by bacteria and certain parasites. It is used to treat bacterial pneumonia, acne, chlamydia infections, Lyme disease, cholera, typhus, an ...
and possibly metronidazole
Metronidazole, sold under the brand name Flagyl among others, is an antibiotic and antiprotozoal medication. It is used either alone or with other antibiotics to treat pelvic inflammatory disease, endocarditis, and bacterial vaginosis. It is ef ...
by mouth is recommended. For those who do not improve after three days or who have severe disease, intravenous antibiotics should be used.
Globally, about 106 million cases of chlamydia and 106 million cases of gonorrhea occurred in 2008. The number of cases of PID, however, is not clear. It is estimated to affect about 1.5 percent of young women yearly. In the United States, PID is estimated to affect about one million people each year. A type of intrauterine device
An intrauterine device (IUD), also known as intrauterine contraceptive device (IUCD or ICD) or coil, is a small, often T-shaped birth control device that is inserted into the uterus to prevent pregnancy. IUDs are one form of long-acting rever ...
(IUD) known as the Dalkon shield led to increased rates of PID in the 1970s. Current IUDs are not associated with this problem after the first month.
Signs and symptoms
 Symptoms in PID range from none to severe. If there are symptoms,
Symptoms in PID range from none to severe. If there are symptoms, fever
Fever, also referred to as pyrexia, is defined as having a body temperature, temperature above the human body temperature, normal range due to an increase in the body's temperature Human body temperature#Fever, set point. There is not a single ...
, cervical
In anatomy, cervical is an adjective that has two meanings:
# of or pertaining to any neck.
# of or pertaining to the female cervix: i.e., the ''neck'' of the uterus.
*Commonly used medical phrases involving the neck are
**cervical collar
**cervic ...
motion tenderness, lower abdominal pain
Abdominal pain, also known as a stomach ache, is a symptom
Signs and symptoms are the observed or detectable signs, and experienced symptoms of an illness, injury, or condition. A sign for example may be a higher or lower temperature than ...
, new or different discharge, painful intercourse
Dyspareunia ( ) is painful sexual intercourse due to medical or psychological causes. The term ''dyspareunia'' covers both female dyspareunia and male dyspareunia, but many discussions that use the term without further specification concern the f ...
, uterine
The uterus (from Latin ''uterus'', plural ''uteri'') or womb () is the hollow organ, organ in the female reproductive system, reproductive system of most female mammals, including humans that accommodates the embryonic development, embryonic an ...
tenderness, adnexal tenderness, or irregular menstruation may be noted.
Other complications include endometritis
Endometritis is inflammation of the inner lining of the uterus ( endometrium). Symptoms may include fever, lower abdominal pain, and abnormal vaginal bleeding or discharge. It is the most common cause of infection after childbirth. It is also p ...
, salpingitis
Salpingitis is an infection causing inflammation in the Fallopian tubes (also called ''salpinges''). It is often included in the umbrella term of pelvic inflammatory disease (PID), along with endometritis, oophoritis, myometritis, parametritis, ...
, tubo-ovarian abscess
A tubo-ovarian abscess (TOA) is one of the late complications of pelvic inflammatory disease (PID) and can be life-threatening if the abscess ruptures and results in sepsis. It consists of an encapsulated or confined pocket of pus with defined ...
, pelvic peritonitis
Peritonitis is inflammation of the localized or generalized peritoneum, the lining of the inner wall of the abdomen and cover of the abdominal organs. Symptoms may include severe pain, swelling of the abdomen, fever, or weight loss. One part or ...
, periappendicitis, and perihepatitis
Perihepatitis is inflammation of the serous or peritoneal coating of the liver.
Perihepatitis is often caused by one of the inflammatory disorders of the female upper genital tract, known collectively as pelvic inflammatory disease.
Some patie ...
.
Complications
PID can causescarring
A scar (or scar tissue) is an area of fibrous tissue that replaces normal skin after an injury. Scars result from the biological process of wound repair in the skin, as well as in other organs, and tissues of the body. Thus, scarring is a na ...
inside the reproductive system, which can later cause serious complications, including chronic pelvic pain, infertility
Infertility is the inability of a person, animal or plant to reproduce by natural means. It is usually not the natural state of a healthy adult, except notably among certain eusocial species (mostly haplodiploid insects). It is the normal state ...
, ectopic pregnancy (the leading cause of pregnancy-related deaths in adult females), and other complications of pregnancy. Occasionally, the infection can spread to the peritoneum
The peritoneum is the serous membrane forming the lining of the abdominal cavity or coelom in amniotes and some invertebrates, such as annelids. It covers most of the intra-abdominal (or coelomic) organs, and is composed of a layer of mesoth ...
causing inflammation and the formation of scar tissue on the external surface of the liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
(Fitz-Hugh–Curtis syndrome
Fitz-Hugh–Curtis syndrome is a rare complication of pelvic inflammatory disease (PID) involving liver capsule inflammation leading to the creation of adhesions. The condition is named after the two physicians, Thomas Fitz-Hugh, Jr and Arthur Ha ...
).
Cause
''Chlamydia trachomatis'' and ''Neisseria gonorrhoeae'' are usually the main cause of PID. Data suggest that PID is often polymicrobial. Isolatedanaerobes
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenate ...
and facultative microorganisms have been obtained from the upper genital tract. ''N. gonorrhoeae'' has been isolated from fallopian tubes, facultative and anaerobic organisms were recovered from endometrial tissues.
The anatomical structure of the internal organs and tissues of the female reproductive tract provides a pathway for pathogens to ascend from the vagina to the pelvic cavity thorough the infundibulum An infundibulum (Latin for ''funnel''; plural, ''infundibula'') is a funnel-shaped cavity or organ.
Anatomy
* Brain: the pituitary stalk, also known as the ''infundibulum'' and ''infundibular stalk'', is the connection between the hypothalamus and ...
. The disturbance of the naturally occurring vaginal microbiota
Microbiota are the range of microorganisms that may be commensal, symbiotic, or pathogenic found in and on all multicellular organisms, including plants. Microbiota include bacteria, archaea, protists, fungi, and viruses, and have been found t ...
associated with bacterial vaginosis
Bacterial vaginosis (BV) is a disease of the vagina caused by excessive growth of bacteria. Common symptoms include increased vaginal discharge that often smells like fish. The discharge is usually white or gray in color. Burning with urination ...
increases the risk of PID.
''N. gonorrhoea'' and ''C. trachomati''s are the most common organisms. The least common were infections caused exclusively by anaerobes and facultative organisms. Anaerobes and facultative bacteria were also isolated from 50 percent of the patients from whom ''Chlamydia'' and ''Neisseria'' were recovered; thus, anaerobes and facultative bacteria were present in the upper genital tract of nearly two-thirds of the PID patients. PCR and serological tests have associated extremely fastidious organism with endometritis, PID, and tubal factor infertility. Microorganisms associated with PID are listed below.
Rarely cases of PID have developed in people who have stated they have never had sex.
Bacteria
*''Chlamydia trachomatis
''Chlamydia trachomatis'' (), commonly known as chlamydia, is a bacterium that causes chlamydia, which can manifest in various ways, including: trachoma, lymphogranuloma venereum, nongonococcal urethritis, cervicitis, salpingitis, pelvic inflamma ...
''
*''Neisseria gonorrhoeae
''Neisseria gonorrhoeae'', also known as ''gonococcus'' (singular), or ''gonococci'' (plural), is a species of Gram-negative diplococci bacteria isolated by Albert Ludwig Sigesmund Neisser, Albert Neisser in 1879. It causes the sexually transmit ...
''
*''Prevotella
''Prevotella'' is a genus of Gram-negative bacteria.
''Prevotella'' spp. are members of the oral, vaginal, and gut microbiota and are often recovered from anaerobic infections of the respiratory tract. These infections include aspiration pneum ...
'' spp.
*''Streptococcus pyogenes
''Streptococcus pyogenes'' is a species of Gram-positive, aerotolerant bacteria in the genus ''Streptococcus''. These bacteria are extracellular, and made up of non-motile and non-sporing cocci (round cells) that tend to link in chains. They are ...
''
*''Prevotella bivia
''Prevotella bivia'' is a species of bacteria in the genus ''Prevotella''. It is gram-negative. It is one cause of pelvic inflammatory disease.
Other ''Prevotella'' spp. are members of the oral and vaginal microbiota, and are recovered fro ...
''
*''Prevotella disiens
''Prevotella'' is a genus of Gram-negative bacteria.
''Prevotella'' spp. are members of the oral, List of microbiota species of the lower reproductive tract of women, vaginal, and gut microflora, gut microbiota and are often recovered from ana ...
''
*''Bacteroides
''Bacteroides'' is a genus of Gram-negative, obligate anaerobic bacteria. ''Bacteroides'' species are non endospore-forming bacilli, and may be either motile or nonmotile, depending on the species. The DNA base composition is 40–48% GC. Unusu ...
'' spp.
*'' Peptostreptococcus asaccharolyticus''
*''Peptostreptococcus anaerobius
''Peptostreptococcus anaerobius'' is a species of bacteria belonging to the ''Peptostreptococcus'' genus of anaerobic, Gram-positive, non- spore forming bacteria. The cells are small, spherical, and can occur in short chains, in pairs or individ ...
''
*''Gardnerella vaginalis
''Gardnerella vaginalis'' is a species of Gram-variable-staining facultative anaerobic bacteria. The organisms are small (1.0–1.5 μm in diameter) non-spore-forming, nonmotile coccobacilli.
Once classified as ''Haemophilus vaginalis'' an ...
''
*''Escherichia coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escher ...
''
*Group B streptococcus
''Streptococcus agalactiae'' (also known as group B streptococcus or GBS) is a gram-positive coccus (round bacterium) with a tendency to form chains (as reflected by the genus name '' Streptococcus''). It is a beta- hemolytic, catalase-negative ...
* α-hemolytic streptococcus
* Coagulase-negative staphylococcus
*''Atopobium vaginae
''Fannyhessea vaginae'' is a species of bacteria in the family ''Atopobiaceae''. It is a facultative anaerobic, Gram-positive rod-shaped or elliptical coccobacilli found as single elements or in pairs or short chains. It is typically isolated fr ...
''
*''Acinetobacter
''Acinetobacter'' is a genus of gram-negative bacteria belonging to the wider class of Gammaproteobacteria. ''Acinetobacter'' species are oxidase-negative, exhibit twitching motility, and occur in pairs under magnification.
They are important ...
'' spp.
*'' Dialister'' spp.
*''Fusobacterium gonidiaformans
''Fusobacterium'' is a genus of anaerobic, Gram-negative, non-sporeforming bacteria belonging to Gracilicutes. Individual cells are slender, rod-shaped bacilli with pointed ends.
Strains of ''Fusobacterium'' cause several human diseases, includin ...
''
*''Gemella
''Gemella'' is a genus of Gram-positive bacteria that thrive best at high partial pressure of CO2.
Description
A Gemella species was first described as Neisseria hemolysans in 1938. It was reclassified as a new genus in 1960 when strains were ...
'' spp.
*'' Leptotrichia'' spp.
*''Mogibacterium
''Mogibacterium'' is a Gram-positive, strictly anaerobic and non- spore-forming bacterial genus from the family of Eubacteriaceae
The Eubacteriaceae are a family of Gram-positive bacteria in the order Clostridiales.
Phylogeny
The currently a ...
'' spp.
*''Porphyromonas
''Porphyromonas'' is a Gram-negative, non-spore-forming, obligately anaerobic and non-motile genus from the family of Porphyromonadaceae. There were 16 different Porphyromonas species documented as of 2015 which reside in both animal and human r ...
'' spp.
*''Sphingomonas
''Sphingomonas'' was defined in 1990 as a group of Gram-negative, rod-shaped, chemoheterotrophic, strictly aerobic bacteria. They possess ubiquinone 10 as their major respiratory quinone, contain glycosphingolipids (GSLs), specifically ceramide ...
'' spp.
*''Veillonella
''Veillonella'' are Gram-negative bacteria (Gram stain pink) anaerobic cocci, unlike most Bacillota, which are Gram-positive bacteria. This bacterium is well known for its lactate fermenting abilities. It is a normal bacterium in the intestine ...
'' spp.
*''Cutibacterium acnes
''Cutibacterium acnes'' (formerly ''Propionibacterium acnes'') is the relatively slow-growing, typically aerotolerant Anaerobic organism, anaerobic, gram-positive bacterium (rod) linked to the skin condition of acne vulgaris, acne; it can also ca ...
''
*''Mycoplasma genitalium
''Mycoplasma genitalium'' (''MG'', commonly known as Mgen) is a sexually transmitted, small and pathogenic bacterium that lives on the mucous epithelial cells of the urinary and genital tracts in humans. Medical reports published in 2007 and 201 ...
''
*''Mycoplasma hominis
''Mycoplasma hominis'' is a species of bacteria in the genus ''Mycoplasma''. ''M.hominis'' has the ability to penetrate the interior of human cells. Along with ureaplasmas, mycoplasmas are the smallest free-living organisms known.
They have no c ...
''
*''Ureaplasma
''Ureaplasma'' is a genus of bacteria belonging to the family Mycoplasmataceae. As the name imples, ''Ureaplasma'' is urease positive.
Phylogeny
The currently accepted taxonomy is based on the List of Prokaryotic names with Standing in Nomenc ...
'' spp.
Diagnosis

 Upon a
Upon a pelvic examination
A pelvic examination is the physical examination of the external and internal female pelvic organs. It is frequently used in gynecology for the evaluation of symptoms affecting the female reproductive and urinary tract, such as pain, bleeding, dis ...
, cervical motion, uterine
The uterus (from Latin ''uterus'', plural ''uteri'') or womb () is the hollow organ, organ in the female reproductive system, reproductive system of most female mammals, including humans that accommodates the embryonic development, embryonic an ...
, or adnexal tenderness will be experienced. Mucopurulent cervicitis
Cervicitis is inflammation of the uterine cervix. Cervicitis in women has many features in common with urethritis in men and many cases are caused by sexually transmitted infections. Non-infectious causes of cervicitis can include intrauterine d ...
and or urethritis
Urethritis is the inflammation of the urethra. The most common symptoms include painful or difficult urination and urethral discharge. It is a commonly treatable condition usually caused by infection with bacteria. This bacterial infection is oft ...
may be observed. In severe cases more testing may be required such as laparoscopy
Laparoscopy () is an operation performed in the abdomen or pelvis using small incisions (usually 0.5–1.5 cm) with the aid of a camera. The laparoscope aids diagnosis or therapeutic interventions with a few small cuts in the abdomen.Medli ...
, intra-abdominal bacteria sampling and culturing, or tissue biopsy.
Laparoscopy can visualize "violin-string" adhesion
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another).
The forces that cause adhesion and cohesion can be ...
s, characteristic of Fitz-Hugh–Curtis perihepatitis and other abscesses that may be present.
Other imaging methods, such as ultrasonography, computed tomography (CT), and magnetic imaging (MRI), can aid in diagnosis. Blood tests can also help identify the presence of infection: the erythrocyte sedimentation rate (ESR), the C-reactive protein (CRP) level, and chlamydial and gonococcal DNA probes.
Nucleic acid amplification tests (NAATs), direct fluorescein tests (DFA), and enzyme-linked immunosorbent assays (ELISA) are highly sensitive tests that can identify specific pathogens present. Serology testing for antibodies is not as useful since the presence of the microorganisms in healthy people can confound interpreting the antibody titer levels, although antibody levels can indicate whether an infection is recent or long-term.
Definitive criteria include histopathologic
Histopathology (compound of three Greek words: ''histos'' "tissue", πάθος ''pathos'' "suffering", and -λογία ''-logia'' "study of") refers to the microscopic examination of tissue in order to study the manifestations of disease. Spec ...
evidence of endometritis, thickened filled Fallopian tubes
The fallopian tubes, also known as uterine tubes, oviducts or salpinges (singular salpinx), are paired tubes in the human female that stretch from the uterus to the ovaries. The fallopian tubes are part of the female reproductive system. In ot ...
, or laparoscopic
Laparoscopy () is an operation performed in the abdomen or pelvis using small incisions (usually 0.5–1.5 cm) with the aid of a camera. The laparoscope aids diagnosis or therapeutic interventions with a few small cuts in the abdomen.Medli ...
findings. Gram stain
In microbiology and bacteriology, Gram stain (Gram staining or Gram's method), is a method of staining used to classify bacterial species into two large groups: gram-positive bacteria and gram-negative bacteria. The name comes from the Danish ...
/smear becomes definitive in the identification of rare, atypical and possibly more serious organisms. Two thirds of patients with laparoscopic evidence of previous PID were not aware they had PID, but even asymptomatic PID can cause serious harm.
Laparoscopic identification is helpful in diagnosing tubal disease; a 65 percent to 90 percent positive predictive value
The positive and negative predictive values (PPV and NPV respectively) are the proportions of positive and negative results in statistics and diagnostic tests that are true positive and true negative results, respectively. The PPV and NPV descr ...
exists in patients with presumed PID.
Upon gynecologic ultrasound
Gynecologic ultrasonography or gynecologic sonography refers to the application of medical ultrasonography to the female pelvic organs (specifically the uterus, the ovaries, and the fallopian tubes) as well as the bladder, the adnexa, and the rec ...
, a potential finding is ''tubo-ovarian complex'', which is edema
Edema, also spelled oedema, and also known as fluid retention, dropsy, hydropsy and swelling, is the build-up of fluid in the body's Tissue (biology), tissue. Most commonly, the legs or arms are affected. Symptoms may include skin which feels t ...
tous and dilated pelvic structures as evidenced by vague margins, but without abscess
An abscess is a collection of pus that has built up within the tissue of the body. Signs and symptoms of abscesses include redness, pain, warmth, and swelling. The swelling may feel fluid-filled when pressed. The area of redness often extends b ...
formation.
Differential diagnosis
A number of other causes may produce similar symptoms including appendicitis, ectopic pregnancy, hemorrhagic or rupturedovarian cysts
An ovarian cyst is a fluid-filled sac within the ovary. Often they cause no symptoms. Occasionally they may produce bloating, lower abdominal pain, or lower back pain. The majority of cysts are harmless. If the cyst either breaks open or causes ...
, ovarian torsion
Ovarian torsion (OT) or adnexal torsion is an abnormal condition where an ovary twists on its attachment to other structures, such that blood flow is decreased. Symptoms typically include pelvic pain on one side. While classically the pain is su ...
, and endometriosis and gastroenteritis
Gastroenteritis, also known as infectious diarrhea and gastro, is an inflammation of the gastrointestinal tract including the stomach and intestine. Symptoms may include diarrhea, vomiting, and abdominal pain. Fever, lack of energy, and dehydra ...
, peritonitis, and bacterial vaginosis among others.
Pelvic inflammatory disease is more likely to reoccur when there is a prior history of the infection, recent sexual contact, recent onset of menses
Menstruation (also known as a period, among other colloquial terms) is the regular discharge of blood and mucosal tissue from the inner lining of the uterus through the vagina. The menstrual cycle is characterized by the rise and fall of horm ...
, or an IUD
An intrauterine device (IUD), also known as intrauterine contraceptive device (IUCD or ICD) or coil, is a small, often T-shaped birth control device that is inserted into the uterus to prevent pregnancy. IUDs are one form of long-acting rever ...
(intrauterine device) in place or if the partner has a sexually transmitted infection
Sexually transmitted infections (STIs), also referred to as sexually transmitted diseases (STDs) and the older term venereal diseases, are infections that are Transmission (medicine), spread by Human sexual activity, sexual activity, especi ...
.
Acute pelvic inflammatory disease is highly unlikely when recent intercourse has not taken place or an IUD is not being used. A sensitive serum pregnancy test
A pregnancy test is used to determine whether a female is pregnant or not. The two primary methods are testing for the female pregnancy hormone (human chorionic gonadotropin (hCG)) in blood or urine using a pregnancy test kit, and scanning with ...
is typically obtained to rule out ectopic pregnancy. Culdocentesis
Culdocentesis is a medical procedure involving the extraction of fluid from the pouch of Douglas (a rectouterine pouch posterior to the vagina) through a needle. It can be one diagnostic technique used in identifying pelvic inflammatory disease ( ...
will differentiate hemoperitoneum
Hemoperitoneum (also haemoperitoneum, sometimes also hematoperitoneum) is the presence of blood in the peritoneal cavity. The blood accumulates in the space between the inner lining of the abdominal wall and the internal abdominal organs. Hemoper ...
(ruptured ectopic pregnancy or hemorrhagic cyst) from pelvic sepsis
Sepsis, formerly known as septicemia (septicaemia in British English) or blood poisoning, is a life-threatening condition that arises when the body's response to infection causes injury to its own tissues and organs. This initial stage is follo ...
(salpingitis, ruptured pelvic abscess, or ruptured appendix).
Pelvic and vaginal ultrasounds are helpful in the diagnosis of PID. In the early stages of infection, the ultrasound may appear normal. As the disease progresses, nonspecific findings can include free pelvic fluid, endometrial thickening, uterine cavity distension by fluid or gas. In some instances the borders of the uterus and ovaries appear indistinct. Enlarged ovaries accompanied by increased numbers of small cysts correlates with PID.
Laparoscopy is infrequently used to diagnose pelvic inflammatory disease since it is not readily available. Moreover, it might not detect subtle inflammation of the fallopian tubes, and it fails to detect endometritis. Nevertheless, laparoscopy is conducted if the diagnosis is not certain or if the person has not responded to antibiotic therapy after 48 hours.
No single test has adequate sensitivity and specificity to diagnose pelvic inflammatory disease. A large multisite U.S. study found that cervical motion tenderness as a minimum clinical criterion increases the sensitivity of the CDC
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency, under the Department of Health and Human Services, and is headquartered in Atlanta, Georgi ...
diagnostic criteria from 83 percent to 95 percent. However, even the modified 2002 CDC criteria do not identify women with subclinical disease.
Prevention
Regular testing forsexually transmitted infections
Sexually transmitted infections (STIs), also referred to as sexually transmitted diseases (STDs) and the older term venereal diseases, are infections that are spread by sexual activity, especially vaginal intercourse, anal sex, and oral se ...
is encouraged for prevention. The risk of contracting pelvic inflammatory disease can be reduced by the following:
* Using barrier methods
Safe sex is sexual activity using methods or contraceptive devices (such as condoms) to reduce the risk of transmitting or acquiring sexually transmitted infections (STIs), especially HIV. "Safe sex" is also sometimes referred to as safer se ...
such as condoms
A condom is a sheath-shaped barrier device used during sexual intercourse to reduce the probability of pregnancy or a sexually transmitted infection (STI). There are both male and female condoms. With proper use—and use at every act of inte ...
; see human sexual behaviour
Human sexual activity, human sexual practice or human sexual behaviour is the manner in which humans experience and express their sexuality. People engage in a variety of sexual acts, ranging from activities done alone (e.g., masturbation) ...
for other listings.
* Seeking medical attention if you are experiencing symptoms of PID.
* Using hormonal combined contraceptive pills also helps in reducing the chances of PID by thickening the cervical mucosal plug & hence preventing the ascent of causative organisms from the lower genital tract.
* Seeking medical attention after learning that a current or former sex partner has, or might have had a sexually transmitted infection.
* Getting a STI history from your current partner and strongly encouraging they be tested and treated before intercourse.
* Diligence in avoiding vaginal activity, particularly intercourse, after the end of a pregnancy (delivery, miscarriage
Miscarriage, also known in medical terms as a spontaneous abortion and pregnancy loss, is the death of an embryo or fetus before it is able to survive independently. Miscarriage before 6 weeks of gestation is defined by ESHRE as biochemical lo ...
, or abortion
Abortion is the termination of a pregnancy by removal or expulsion of an embryo or fetus. An abortion that occurs without intervention is known as a miscarriage or "spontaneous abortion"; these occur in approximately 30% to 40% of pregn ...
) or certain gynecological procedures, to ensure that the cervix closes.
* Reducing the number of sexual partners.
* Sexual monogamy
Monogamy ( ) is a form of dyadic relationship in which an individual has only one partner during their lifetime. Alternately, only one partner at any one time (serial monogamy) — as compared to the various forms of non-monogamy (e.g., polyga ...
.
* Abstinence
Abstinence is a self-enforced restraint from indulging in bodily activities that are widely experienced as giving pleasure. Most frequently, the term refers to sexual abstinence, but it can also mean abstinence from alcohol, drugs, food, etc.
...
Treatment
Treatment is often started without confirmation of infection because of the serious complications that may result from delayed treatment. Treatment depends on theinfectious agent
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
and generally involves the use of antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
therapy although there is no clear evidence of which antibiotic regimen is more effective and safe in the management of PID. If there is no improvement within two to three days, the patient is typically advised to seek further medical attention. Hospitalization sometimes becomes necessary if there are other complications. Treating sexual partners for possible STIs can help in treatment and prevention.
For women with PID of mild to moderate severity, parenteral and oral therapies appear to be effective. It does not matter to their short- or long-term outcome whether antibiotics are administered to them as inpatients or outpatients. Typical regimens include cefoxitin
Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped wi ...
or cefotetan
Cefotetan is an injectable antibiotic of the cephamycin type for prophylaxis and treatment of bacterial infections. It is often grouped together with second-generation cephalosporins and has a similar antibacterial spectrum, but with additional ...
plus doxycycline
Doxycycline is a broad-spectrum tetracycline class antibiotic used in the treatment of infections caused by bacteria and certain parasites. It is used to treat bacterial pneumonia, acne, chlamydia infections, Lyme disease, cholera, typhus, an ...
, and clindamycin
Clindamycin is an antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media (middle ear infec ...
plus gentamicin
Gentamicin is an antibiotic used to treat several types of bacterial infections. This may include bone infections, endocarditis, pelvic inflammatory disease, meningitis, pneumonia, urinary tract infections, and sepsis among others. It is not e ...
. An alternative parenteral regimen is ampicillin
Ampicillin is an antibiotic used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B strepto ...
/sulbactam
Sulbactam is a β-lactamase inhibitor. This drug is given in combination with β-lactam antibiotics to inhibit β-lactamase, an enzyme produced by bacteria that destroys the antibiotics.
It was patented in 1977 and approved for medical use in 19 ...
plus doxycycline. Erythromycin
Erythromycin is an antibiotic used for the treatment of a number of bacterial infections. This includes respiratory tract infections, skin infections, chlamydia infections, pelvic inflammatory disease, and syphilis. It may also be used duri ...
-based medications can also be used. A single study suggests superiority of azithromycin over doxycycline. Another alternative is to use a parenteral regimen with ceftriaxone
Ceftriaxone, sold under the brand name Rocephin, is a third-generation cephalosporin antibiotic used for the treatment of a number of bacterial infections. These include middle ear infections, endocarditis, meningitis, pneumonia, bone and joint ...
or cefoxitin plus doxycycline. Clinical experience guides decisions regarding transition from parenteral to oral therapy, which usually can be initiated within 24–48 hours of clinical improvement.
Prognosis
Even when the PID infection is cured, effects of the infection may be permanent. This makes early identification essential. Treatment resulting in cure is very important in the prevention of damage to thereproductive system
The reproductive system of an organism, also known as the genital system, is the biological system made up of all the anatomical organs involved in sexual reproduction. Many non-living substances such as fluids, hormones, and pheromones are als ...
. Formation of scar tissue due to one or more episodes of PID can lead to tubal blockage, increasing the risk of the inability to get pregnant and long-term pelvic/abdominal pain. Certain occurrences such as a post pelvic operation, the period of time immediately after childbirth (postpartum
The postpartum (or postnatal) period begins after childbirth and is typically considered to end within 6 weeks as the mother's body, including hormone levels and uterus size, returns to a non-pregnant state. The terms puerperium, puerperal perio ...
), miscarriage
Miscarriage, also known in medical terms as a spontaneous abortion and pregnancy loss, is the death of an embryo or fetus before it is able to survive independently. Miscarriage before 6 weeks of gestation is defined by ESHRE as biochemical lo ...
or abortion
Abortion is the termination of a pregnancy by removal or expulsion of an embryo or fetus. An abortion that occurs without intervention is known as a miscarriage or "spontaneous abortion"; these occur in approximately 30% to 40% of pregn ...
increase the risk of acquiring another infection leading to PID.
Epidemiology
Globally about 106 million cases of chlamydia and 106 million cases of gonorrhea occurred in 2008. The number of cases of PID; however, is not clear. It is estimated to affect about 1.5 percent of young women yearly. In the United States PID is estimated to affect about one million people yearly. Rates are highest with teenagers and first time mothers. PID causes over 100,000 women to becomeinfertile
Infertility is the inability of a person, animal or plant to reproduce by natural means. It is usually not the natural state of a healthy adult, except notably among certain eusocial species (mostly haplodiploid insects). It is the normal state ...
in the US each year.
References
External links
CDC
{{Authority control Abdominal pain Sexually transmitted diseases and infections Bacterial diseases Chlamydia infections Infections with a predominantly sexual mode of transmission Wikipedia medicine articles ready to translate Inflammatory diseases of female pelvic organs Mycoplasma Gonorrhea