Noyori asymmetric hydrogenation on:
[Wikipedia]
[Google]
[Amazon]
In 
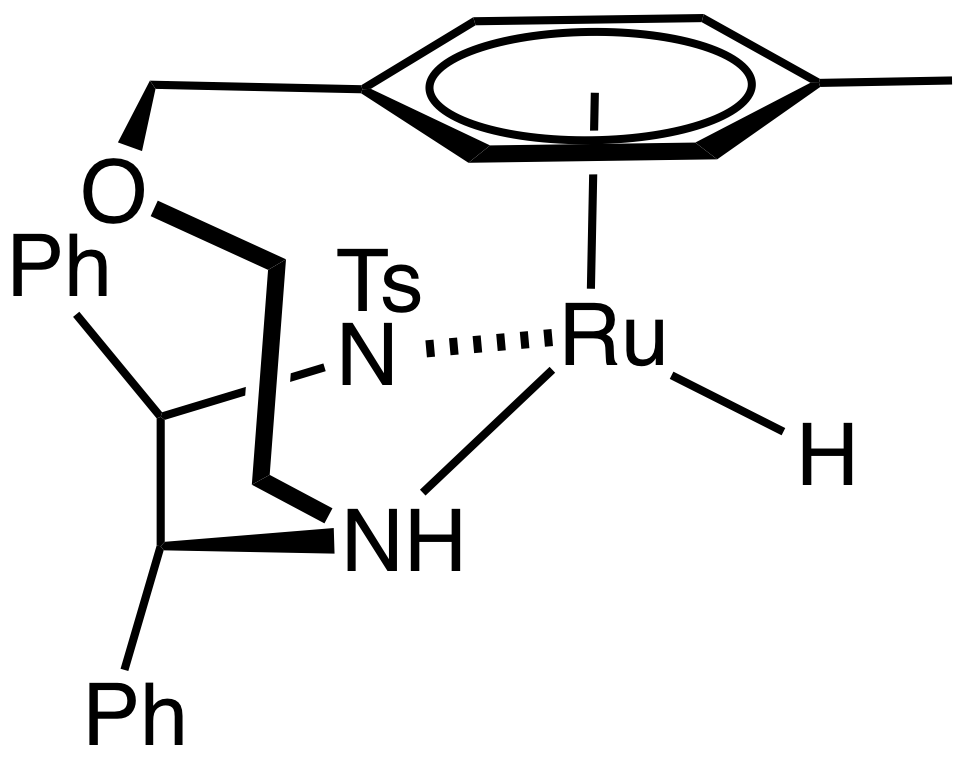
 The BINAP-Ru-diamine dihalide precatalyst is converted to the catalysts by reaction of H2 in the presence of base:
:RuCl2(BINAP)(diamine) + 2 KOBu-t + 2 H2 → RuH2(BINAP)(diamine) + 2 KCl + 2 HOBu-t
The resulting catalysts have three kinds of ligands:
*hydrides, which transfer to the unsaturated substrate
*diamines, which interact with substrate and with base activator by the second coordination sphere
*diphosphine, which confers asymmetry.
The Noyori-class of catalysts are often referred to as bifunctional catalysts to emphasize the fact that both the metal and the (amine) ligand are functional. The mechanism was long assumed to operate by a six membered pericyclic
The BINAP-Ru-diamine dihalide precatalyst is converted to the catalysts by reaction of H2 in the presence of base:
:RuCl2(BINAP)(diamine) + 2 KOBu-t + 2 H2 → RuH2(BINAP)(diamine) + 2 KCl + 2 HOBu-t
The resulting catalysts have three kinds of ligands:
*hydrides, which transfer to the unsaturated substrate
*diamines, which interact with substrate and with base activator by the second coordination sphere
*diphosphine, which confers asymmetry.
The Noyori-class of catalysts are often referred to as bifunctional catalysts to emphasize the fact that both the metal and the (amine) ligand are functional. The mechanism was long assumed to operate by a six membered pericyclic

 Newer routes focus on the hydrogenation of (R)-
Newer routes focus on the hydrogenation of (R)-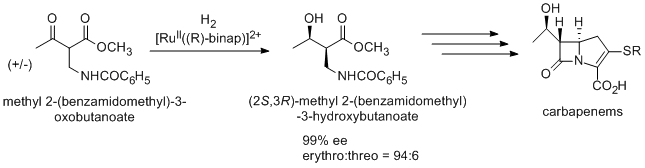 An antipsychotic agent BMS 181100 is synthesized using BINAP/diamine-Ru catalyst.
An antipsychotic agent BMS 181100 is synthesized using BINAP/diamine-Ru catalyst.

chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, the Noyori asymmetric hydrogenation refers to methodology for enantioselective reduction of ketones and related functional groups. This methodology was introduced by Ryoji Noyori, who shared the Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
in 2001 for contributions to asymmetric hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of Hydrogen atom, hydrogen to a target (substrate) molecule with three-dimensional Enantioselective synthesis, spatial selectivity. Critically, this selectivity does not come from ...
. These hydrogenations are used in the production of several drugs, such as the antibacterial levofloxin, the antibiotic carbapenem, and the antipsychotic agent BMS181100.

History
The stoichiometric asymmetric reduction of ketones has long been known, e.g., using boron hydrides. The catalytic asymmetric hydrogenation ofketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
was demonstrated with catalysts based on BINAP
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1� ...
-Ru halides and carboxylates.
Even though the BINAP-Ru dihalide catalyst could reduce functionalized ketones, the hydrogenation of simple ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
remained an unsolved. This challenge was solved with precatalysts of the type RuCl2(diphosphane
Diphosphane, or diphosphine, is an inorganic compound with the chemical formula P2H4. This colourless liquid is one of several binary phosphorus hydrides. It is the impurity that typically causes samples of phosphine to ignite in air.
Propert ...
)(diamine). These catalysts preferentially reduce ketones and aldehydes, leaving olefins and many other substituents unaffected.

Mechanism
 The BINAP-Ru-diamine dihalide precatalyst is converted to the catalysts by reaction of H2 in the presence of base:
:RuCl2(BINAP)(diamine) + 2 KOBu-t + 2 H2 → RuH2(BINAP)(diamine) + 2 KCl + 2 HOBu-t
The resulting catalysts have three kinds of ligands:
*hydrides, which transfer to the unsaturated substrate
*diamines, which interact with substrate and with base activator by the second coordination sphere
*diphosphine, which confers asymmetry.
The Noyori-class of catalysts are often referred to as bifunctional catalysts to emphasize the fact that both the metal and the (amine) ligand are functional. The mechanism was long assumed to operate by a six membered pericyclic
The BINAP-Ru-diamine dihalide precatalyst is converted to the catalysts by reaction of H2 in the presence of base:
:RuCl2(BINAP)(diamine) + 2 KOBu-t + 2 H2 → RuH2(BINAP)(diamine) + 2 KCl + 2 HOBu-t
The resulting catalysts have three kinds of ligands:
*hydrides, which transfer to the unsaturated substrate
*diamines, which interact with substrate and with base activator by the second coordination sphere
*diphosphine, which confers asymmetry.
The Noyori-class of catalysts are often referred to as bifunctional catalysts to emphasize the fact that both the metal and the (amine) ligand are functional. The mechanism was long assumed to operate by a six membered pericyclic transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked wi ...
/intermediate whereby the hydrido ruthenium hydride center (''H''Ru-N''H'') interacts with the carbonyl substrate R2''C''=''O''. DFT and experimental studies have shown that this model is largely incorrect. Instead, the amine backbone interacts strongly with the base activator, which often is used in large excess.
Substrate scope
The BINAP/diamine-Ru catalyst is effective for the asymmetric reduction of both functionalized and simple ketones, and BINAP/diamine-Ru catalyst can catalyzearomatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
, heteroaromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturat ...
, and olefinic ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
enantioselectively. Better stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
is achieved when one substituent is larger than the other (see Flippin-Lodge angle).

Industrial applications
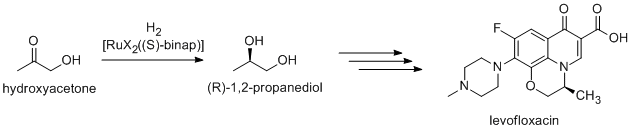
Noyori-inspired hydrogenation catalysts have been applied to the commercial synthesis of number of fine chemicals. (R)-1,2-Propandiol, precursor to the antibacteriallevofloxacin
Levofloxacin, sold under the brand name Levaquin among others, is an antibiotic medication. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori (in combination with other medications), ...
, can be efficiently synthesized from hydroxyacetone using Noyori asymmetric hydrogenation:  Newer routes focus on the hydrogenation of (R)-
Newer routes focus on the hydrogenation of (R)-methyl lactate
Methyl lactate, also known as lactic acid methyl ester, is the organic compound with the formula CH3CH(OH)CO2CH3. It is the methyl ester of lactic acid. A colorless liquid, it is the simplest chiral ester. Being naturally derived, it is readily ...
.
An antibiotic carbapenem
Carbapenems are a class of very effective antibiotic agents most commonly used for the treatment of severe bacterial infections. This class of antibiotics is usually reserved for known or suspected multidrug-resistant (MDR) bacterial infections. ...
is also prepared using Noyori asymmetric hydrogenation via (2S,3R)-methyl 2-(benzamidomethyl)-3-hydroxybutanoate, which is synthesized from racemic methyl 2-(benzamidomethyl)-3-oxobutanoate by dynamic kinetic resolution
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resul ...
.
 An antipsychotic agent BMS 181100 is synthesized using BINAP/diamine-Ru catalyst.
An antipsychotic agent BMS 181100 is synthesized using BINAP/diamine-Ru catalyst.

See also
* Midland Alpine Borane Reduction * Corey-Itsuno reduction *MACHO catalyst
In homogeneous catalysis, MACHO catalysts are metal complexes containing MACHO ligands, which are of the type HN(CH2CH2PR2)2, where R is typically phenyl or isopropyl. Complexes with ruthenium(II) and iridium(III) have received much attention for ...
References
{{reflist Organic reduction reactions Chemical synthesis Stereochemistry Hydrogenation Name reactions Hydrogenation, Noyori