A persistent carbene (also known as stable carbene) is a type of
carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC)
(sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R
2N)
2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give
heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
carbenes, such as those derived from
imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
,
imidazoline
Imidazoline is a class of heterocycles formally derived from imidazoles by the reduction of one of the two double bonds. Three isomers are known, 2-imidazolines, 3-imidazolines, and 4-imidazolines. The 2- and 3-imidazolines contain an imine
In ...
,
thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular fo ...
or
triazole.
Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing
dimerization, many can be isolated as pure substances.
Persistent carbenes tend to exist in the
singlet. Their stability is only partly due to
steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
by bulky groups. Some singlet carbenes are
thermodynamically stable and can be isolated and indefinitely stored. Others dimerise slowly over days. Triplet state carbenes have
half-lives measured in seconds, and therefore can be observed but not stored.
History
Early evidence
In 1957,
Ronald Breslow proposed that a relatively stable
nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
carbene, a
thiazol-2-ylidene derivative, was involved in the
catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
of
vitamin B1 (thiamine) that yields
furoin from
furfural.
In this cycle, the vitamin's
thiazolium
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular fo ...
ring exchanges a hydrogen atom (attached to carbon 2 of the ring) for a furfural residue. In
deuterated water, the C2-
proton was found to rapidly exchange for a
deuteron
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one n ...
in a statistical
equilibrium.

This exchange was proposed to proceed via intermediacy of a thiazol-2-ylidene. In 2012 the isolation of the so-called ''Breslow intermediate'' was reported.
In 1960,
Hans-Werner Wanzlick and coworkers conjectured that carbenes derived from
dihydroimidazol-2-ylidene were produced by
vacuum pyrolysis of the corresponding 2-trichloromethyl
dihydroimidazole compounds with the loss of
chloroform.
They conjectured that the carbene existed in equilibrium with its
dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
, a
tetraaminoethylene
In organic chemistry, tetraaminoethylene is a hypothetical, organic compound with formula or . Like all polyamines that are geminal, this compound has never been synthesised and is believed to be extremely unstable.Stephen A. Lawrence (2004) ...
derivative, the so-called
Wanzlick equilibrium. This conjecture was challenged by
Lemal and coworkers in 1964, who presented evidence that the dimer did not dissociate;
and by Winberg in 1965.
However, subsequent experiments by Denk, Herrmann and others have confirmed this equilibrium, albeit in specific circumstances.
Isolation of persistent carbenes
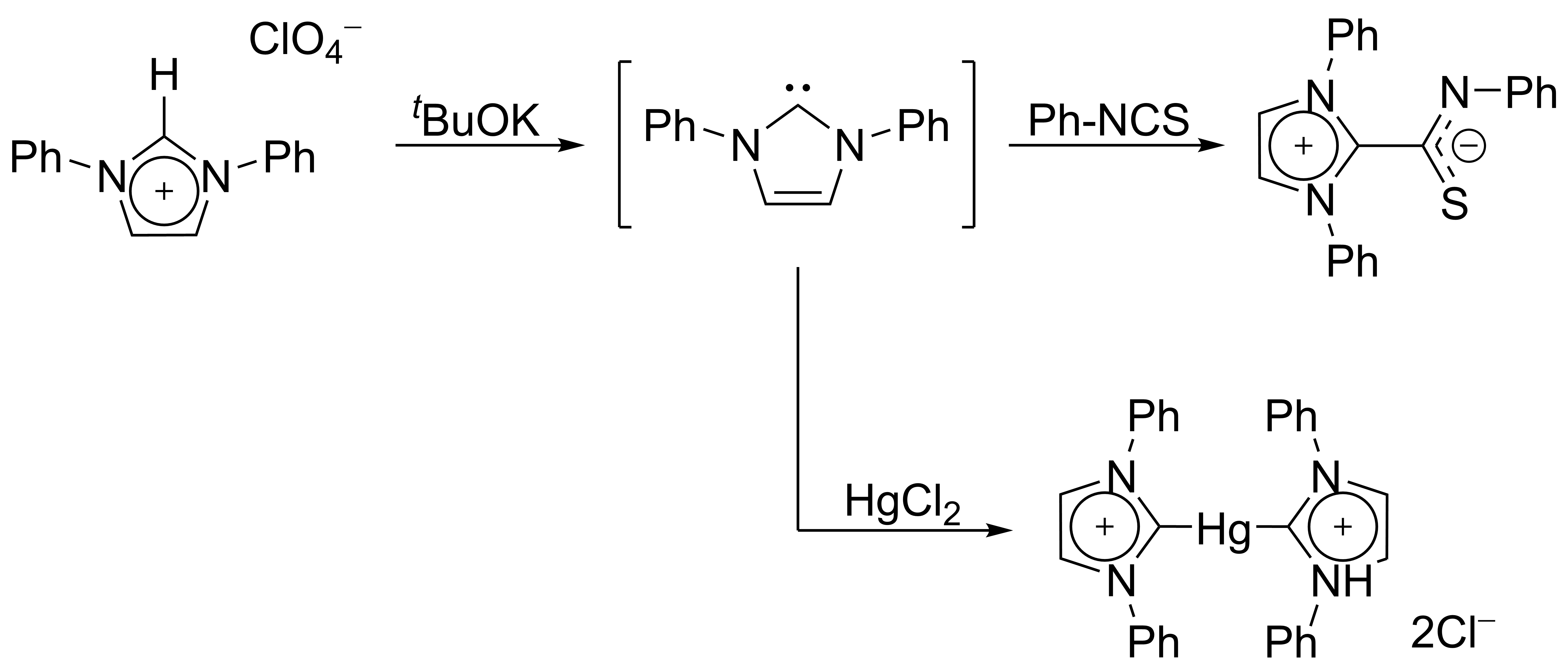
In 1970, Wanzlick's group generated imidazol-2-ylidene carbenes by the deprotonation of an
imidazolium
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
salt.
Wanzlick as well as
Roald Hoffmann
Roald Hoffmann (born Roald Safran; July 18, 1937) is a Polish-American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He has also published plays and poetry. He is the Frank H. T. Rhodes Professor of Humane Letters, Emeritus, at ...
,
proposed that these imidazole-based carbenes should be more stable than their 4,5-dihydro analogues, due to Hückel-type
aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
. Wanzlick did not however isolate imidazol-2-ylidenes, but instead their
coordination compounds with
mercury and
isothiocyanate
In organic chemistry, isothiocyanate is the functional group , formed by substituting the oxygen in the isocyanate group with a sulfur. Many natural isothiocyanates from plants are produced by enzymatic conversion of metabolites called glucosi ...
:
In 1988,
Guy Bertrand and others isolated a
phosphinocarbene. These species can be represented as either a λ
3-phosphinocarbene or λ
5-
phosphaacetylene:

These compounds were called "push-pull carbenes" in reference to the contrasting electron affinities of the phosphorus and silicon atoms. They exhibit both carbenic and
alkynic reactivity. An X-ray structure of this molecule has not been obtained and at the time of publication some doubt remained as to their exact carbenic nature.
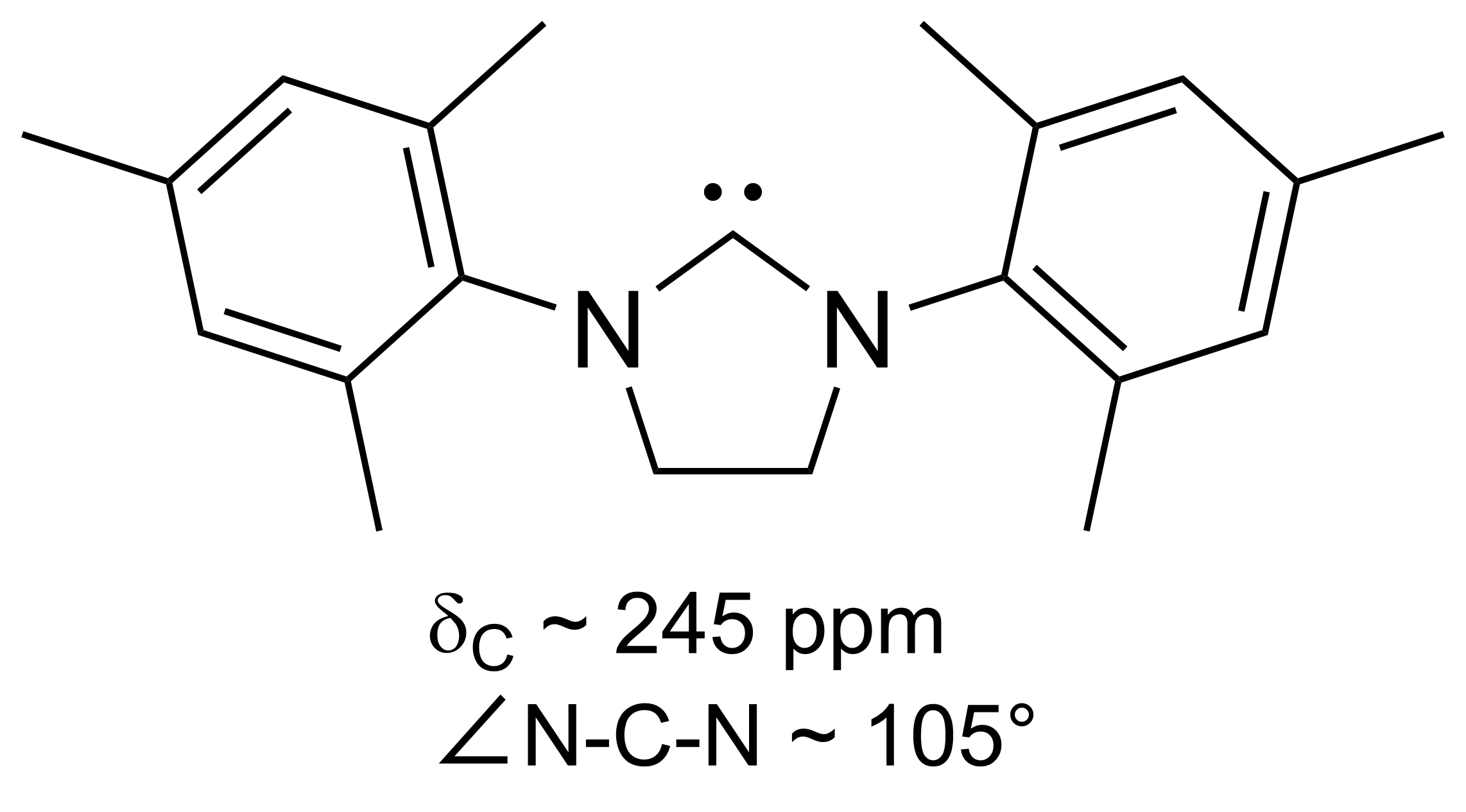
In 1991, Arduengo and coworkers crystallized a diaminocarbene by
deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
of an imidazolium cation:
This carbene, the forerunner of a large family of carbenes with the imidazol-2-ylidene core, is indefinitely stable at room temperature in the absence of oxygen and moisture. It melts at 240–241 °C without decomposition. The
13C NMR spectrum shows a signal at 211 ppm for the carbenic atom. The
X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
structure revealed longer N–C
bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
s in the ring of the carbene than in the parent imidazolium compound, indicating that there was very little
double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
character to these bonds.
The first air-stable ylidic carbene, a chlorinated member of the imidazol-2-ylidene family, was obtained in 1997.
In 2000, Bertrand obtained additional carbenes of the phosphanyl type, including (phosphanyl)(trifluoromethyl)carbene, stable in solution at -30 °C and a moderately stable (amino)(aryl)carbene with only one heteroatom adjacent to the carbenic atom.
Factors affecting stability of heteroatom-stabilized carbenes

Steric hindrance
The stability of Arduengo carbenes was initially attributed to the bulky ''N''-
adamantyl
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the ...
substituents, which prevents the carbene from dimerising due to
steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
. Replacement of the ''N''-adamantyl groups with
methyl groups also affords stable NHCs.
[ Thus, imidazole-2-ylidenes are thermodynamically stable.
]
Aromatic participation
It had been also conjectured that the double bond between carbons 4 and 5 of the imidazolium ring backbone, which gave aromatic character to that system, was important for the carbene's stability. This conjecture was disproved in 1995 by Arduengo's group, who obtained a derivative of dihydroimidazol-2-ylidene, lacking the double bond.[ The thermodynamical stability in this compound, and the role of steric protection in preventing dimerization, has been a topic of some dispute.][
]
Ring participation
 The first acyclic persistent carbene was reported in 1996,
The first acyclic persistent carbene was reported in 1996,[ thus showing that a cyclic backbone was not necessary for their stability. Unlike the cyclic derivatives, the acyclic carbenes are flexible with respect to rotation of the bonds to the carbenic atom. By measuring the barrier to rotation of these bonds, the extent of their ]double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
character could be measured, and the ylidic nature of this carbene could be determined. Like the cyclic diaminocarbenes, unhindered variants tend to dimerize.[
]
Nitrogen participation
Most persistent carbenes are stabilized by two flanking nitrogen centers. The aminothiocarbene and an aminooxycarbene are outliers.[ In these stable compounds, the carbenic atom lies between a nitrogen atom and either a sulfur or oxygen atom:
] Unlike NHCs, these carbenes are not thermodynamically stable but tend to dimerize.
In bis(diisopropylamino)cyclopropenylidene, which is stable at room temperature, the carbene atom is connected to two carbon atoms, in a three-member ring that retains the aromaticity and geometry of the cyclopropenylidene ring. This example demonstrated that the presence of heteroatoms next to the carbene is not necessary for stability, either.
Unlike NHCs, these carbenes are not thermodynamically stable but tend to dimerize.
In bis(diisopropylamino)cyclopropenylidene, which is stable at room temperature, the carbene atom is connected to two carbon atoms, in a three-member ring that retains the aromaticity and geometry of the cyclopropenylidene ring. This example demonstrated that the presence of heteroatoms next to the carbene is not necessary for stability, either.[
]
Molecular orbitals and persistent carbenes
The last example offers a glimpse of an answer to the question why these compounds are (meta)stable after all. It too, offers some sight at the limits of the use of Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
.
= Carbon participation
=
In this case the unoccupied p-orbital on the carbene carbon is not empty at all. It takes part in a molecular orbital occupied by two electrons. The electron density at the carbene centre in this orbital will have an estimated value of 1 (1/3). So the total count of electrons in the carbon valence orbitals will be: 4 in two σ-bonds to the other ring carbons, 2 in the lone pair and 1(1/3) in the aromatic molecular orbital, adding up to 7(1/3), only slightly less than the required 8. So stating the carbene carbon only carries six electrons at least is not accurate.
= Imidazole based carbenes
=
 For the
For the imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
-derived carbenes the same is true. The "unoccupied" p-orbital on the carbene centre is part of an aromatic structure with 6 electrons, 2 from each participating nitrogen p-orbital and 1 from each carbon at the formal double bound, delocalized in a 5 membered ring. Here too, the electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
at the p-orbital of the carbene centre will be somewhat larger than 1. Again, only claiming 6 electrons on the carbene centre at least is not accurate.
= Diamino carbenes
=
120px, MO's of the allylic system.
A saturation on the carbons 4 and 5 of the imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
ring, or the lack of a ring structure all together leaves the carbene centre with two adjacent nitrogen atoms. Drawing the molecule with Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s, allowing only localized bonds indeed leaves the carbene centre with only six electrons. The molecular structure however, contains three atoms in a row, of which two, the nitrogens, possess a lone electron pair and the third one has an empty (!) p-orbital. This opens the road to an allyl like de-localisation of electrons. In the figure at the right, although borrowed from a true allylic molecule, the MO's of such a system are shown. Again it is clear the carbene centre carries electron density in this orbital and again claiming 6 electrons on the carbene centre at least is not accurate.
= Conclusion
=
Now the arguments are set for the final question: why are these carbene like structures (meta)stable at least.
These compounds are called carbenes as their Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons t ...
s only show six electrons on the carbene centre. Reality is, the carbene like carbon atoms in these compounds have a larger electron density than simple Lewis structures predict. Combined with an electron donating environment able to at least partly fill the electron gap on the carbene centre enough energy is found to stabilize the compounds.
Classes of stable carbenes
The following are examples of the classes of stable carbenes isolated to date:
Imidazol-2-ylidenes
The first stable carbenes to be isolated were based on an imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
ring, with the hydrogen in carbon 2 of the ring (between the two nitrogen atoms) removed, and other hydrogens replaced by various groups. These imidazol-2-ylidenes are still the most stable and the most well studied and understood family of persistent carbenes.
A considerable range of imidazol-2-ylidenes have been synthesised, including those in which the 1,3-positions have been functionalised with alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromaticity, aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar ...
,chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
substituents:[
] In particular, substitution of two
In particular, substitution of two chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
atoms for the two hydrogens at ring positions 4 and 5 yielded the first air-stable carbene.[
] Its extra stability probably results from the electron-withdrawing
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of th ...
effect of the chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
substituents, which reduce the electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
on the carbon atom bearing the lone pair, via induction through the sigma-backbone.
Molecules containing two and even three imidazol-2-ylidene groups have also been synthesised.
Triazol-5-ylidenes
Depending on the arrangement of the three nitrogen atoms in triazol-5-ylidene, there are two possible isomers, namely 1,2,3-triazol-5-ylidenes and 1,2,4-triazol-5-ylidenes.
 The triazol-5-ylidenes based on the
The triazol-5-ylidenes based on the 1,2,4-triazole
1,2,4-Triazole (as ligand in coordination compounds, Htrz abbreviation is sometimes used) is one of a pair of isomeric chemical compounds with molecular formula CHN, called triazoles, which have a five-membered ring of two carbon atoms and three n ...
ring are pictured below and were first prepared by Enders and coworkers Triazole-based carbenes are thermodynamically stable and have diagnostic 13C NMR chemical shift values between 210 and 220 ppm for the carbenic carbon. The X-ray structure of the triphenyl substituted carbene above shows an N–C–N bond angle of around 101°. The 5-methoxytriazole precursor to this carbene was made by the treatment of a triazolium salt with sodium methoxide, which attacks as a nucleophile.
Triazole-based carbenes are thermodynamically stable and have diagnostic 13C NMR chemical shift values between 210 and 220 ppm for the carbenic carbon. The X-ray structure of the triphenyl substituted carbene above shows an N–C–N bond angle of around 101°. The 5-methoxytriazole precursor to this carbene was made by the treatment of a triazolium salt with sodium methoxide, which attacks as a nucleophile.[ This may indicate that these carbenes are less aromatic than imidazol-2-ylidenes, as the imidazolium precursors do not react with nucleophiles due to the resultant loss of aromaticity.
]
Other diaminocarbenes
The two families above can be seen as special cases of a broader class of compounds which have a carbenic atom bridging two nitrogen atoms. A range of such diaminocarbenes have been prepared principally by Roger Alder's research group. In some of these compounds, the N–C–N unit is a member of a five- or six-membered non-aromatic ring, Unlike the aromatic imidazol-2-ylidenes or triazol-5-ylidenes, these carbenes appear not to be thermodynamically stable, as shown by the dimerisation of some unhindered cyclic and acyclic examples.
Unlike the aromatic imidazol-2-ylidenes or triazol-5-ylidenes, these carbenes appear not to be thermodynamically stable, as shown by the dimerisation of some unhindered cyclic and acyclic examples.[ Studies][ suggest that these carbenes dimerise via acid catalysed dimerisation (as in the Wanzlick equilibrium).
Diaminocarbenes have diagnostic 13C NMR chemical shift values between 230 and 270 ppm for the carbenic atom. The X-ray structure of dihydroimidazole-2-ylidene shows a N–C–N bond angle of about 106°, whilst the angle of the acyclic carbene is 121°, both greater than those seen for imidazol-2-ylidenes.
]
Heteroamino carbenes
There exist several variants of the stable carbenes above where one of the nitrogen atoms adjacent to the carbene center (the α nitrogens) has been replaced by an alternative heteroatom, such as oxygen, sulfur, or phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
. In particular, the formal substitution of sulfur for one of the nitrogens in imidazole would yield the aromatic heterocyclic compound
In particular, the formal substitution of sulfur for one of the nitrogens in imidazole would yield the aromatic heterocyclic compound thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular fo ...
. A thiazole based carbene (analogous to the carbene postulated by Breslow) has been prepared and characterised by X-ray crystallography.[ Other non-aromatic aminocarbenes with O, S and P atoms adjacent (i.e. alpha) to the carbene centre have been prepared, for example, ]thio-
The prefix thio-, when applied to a chemical, such as an ion, means that an oxygen atom in the compound has been replaced by a sulfur atom. This term is often used in organic chemistry. For example, from the word ''ether,'' referring to an ox ...
and oxyiminium based carbenes have been characterised by X-ray crystallography.[
Since ]oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
and sulfur are divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an chemical element, element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, ...
, steric protection of the carbenic centre is limited especially when the N–C–X unit is part of a ring. These acyclic carbenes have diagnostic 13C NMR chemical shift values between 250 and 300 ppm for the carbenic carbon, further downfield than any other types of stable carbene. X-ray structures have shown N–C–X bond angles of around 104° and 109° respectively.
Carbenes that formally derive from imidazole-2-ylidenes by substitution of sulfur, oxygen, or other chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioac ...
s for ''both'' α-nitrogens are expected to be unstable, as they have the potential to dissociate into an alkyne (R1C≡CR2) and a carbon dichalcogenide (X1=C=X2).
Non-amino carbenes
The reaction of carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical n ...
(CS2) with electron deficient acetylene derivatives is proposed to give transient 1,3-dithiolium carbenes (i.e. where X1 = X2 = S), which then dimerise to give derivatives of tetrathiafulvene
Tetrathiafulvalene is an organosulfur compound with the formula (. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, , by replacement of four CH groups wit ...
. Thus it is possible that the reverse of this process might be occurring in similar carbenes.
Bertrand's carbenes
In Bertrand's persistent carbenes, the unsaturated carbon is bonded to a phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
and a silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
. However, these compounds seem to exhibit some alkynic properties, and when published the exact carbenic nature of these red oils was in debate.[
]
Other nucleophilic carbenes
One stable ''N''-heterocyclic carbeneborazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For thi ...
with one boron atom replaced by a methylene group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms chemical bond, bound to a carbon atom, which is connected to the remainder of the molecule by two single bond, single bonds. The group may be re ...
. This results in a planar six-electron compound.

Cyclopropenylidenes
Another family of carbenes is based on a cyclopropenylidene core, a three-carbon ring with a double bond between the two atoms adjacent to the carbenic one. This family is exemplified by bis(diisopropylamino)cyclopropenylidene.
Triplet state carbenes
In 2001, Hideo Tomioka and his associates were able to produce a comparatively stable triplet carbene ( bis(9-anthryl)carbene, with a half-life of 19 minutes), by taking advantage of electron delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
.
Although the figure below shows the two parts of the molecule in one flat plane, molecular geometry puts the two aromatic parts in orthogonal positions with respect to each other.
 In 2006 a triplet carbene was reported by the same group with a
In 2006 a triplet carbene was reported by the same group with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 40 minutes. This carbene is prepared by a photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
decomposition
Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is e ...
of a diazomethane
Diazomethane is the chemical compound CH2N2, discovered by German chemist Hans von Pechmann in 1894. It is the simplest diazo compound. In the pure form at room temperature, it is an extremely sensitive explosive yellow gas; thus, it is almost ...
precursor by 300 nm light in benzene with expulsion of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
gas.
Again the figure below is not an adequate representation of the actual molecular structure: both phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
rings are positioned orthogonal with respect to each other. The carbene carbon has an sp- hybridisation, the two remaining orthogonal p- orbitals each conjugating with one of the aromatic rings.
 Exposure to oxygen (a triplet diradical) converts this carbene to the corresponding
Exposure to oxygen (a triplet diradical) converts this carbene to the corresponding benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylket ...
. The diphenylmethane compound is formed when it is trapped by cyclohexa-1,4-diene
1,4-Cyclohexadiene is an organic compound with the formula C6H8. It is a colourless, flammable liquid that is of academic interest as a prototype of a large class of related compounds called terpenoids, an example being γ-terpinene. An isomer o ...
. As with the other carbenes, this species contains large bulky substituents, namely bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
and the trifluoromethyl groups on the phenyl rings, that shield the carbene and prevent or slow down the process of dimerization to a 1,1,2,2-tetra(phenyl)alkene. Based on computer simulations
Computer simulation is the process of mathematical modelling, performed on a computer, which is designed to predict the behaviour of, or the outcome of, a real-world or physical system. The reliability of some mathematical models can be dete ...
, the distance
Distance is a numerical or occasionally qualitative measurement of how far apart objects or points are. In physics or everyday usage, distance may refer to a physical length or an estimation based on other criteria (e.g. "two counties over"). ...
of the divalent carbon atom to its neighbors is claimed to be 138 picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer (American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of ...
s with a bond angle
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
of 158.8°. The planes of the phenyl groups are almost at right angles to each other (the dihedral angle
A dihedral angle is the angle between two intersecting planes or half-planes. In chemistry, it is the clockwise angle between half-planes through two sets of three atoms, having two atoms in common. In solid geometry, it is defined as the un ...
being 85.7°).
Mesoionic carbenes
Mesoionic carbenes (MICs) are similar to ''N''-heterocyclic carbenes (NHCs) except that canonical resonance structures with the carbene depicted cannot be drawn without adding additional charges. Mesoionic carbenes are also referred to as abnormal ''N''-heterocyclic carbenes (aNHC) or remote ''N''-heterocyclic carbenes (rNHC). A variety of free carbenes can be isolated and are stable at room temperature. Other free carbenes are not stable and are susceptible to intermolecular decomposition pathways.
Chemical properties
Basicity and nucleophilicity
The imidazol-2-ylidenes are strong bases, having p''K''a ≈ 24 for the conjugate acid in dimethyl sulfoxide (DMSO):[
] However, further work showed that diaminocarbenes will deprotonate the DMSO solvent, with the resulting anion reacting with the resulting amidinium salt.
However, further work showed that diaminocarbenes will deprotonate the DMSO solvent, with the resulting anion reacting with the resulting amidinium salt.
 Reaction of imidazol-2-ylidenes with 1-bromohexane gave 90% of the 2-substituted adduct, with only 10% of the corresponding
Reaction of imidazol-2-ylidenes with 1-bromohexane gave 90% of the 2-substituted adduct, with only 10% of the corresponding alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
, indicating that these molecules are also reasonably nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
.
p''K''a values for the conjugate acids of several NHC families have been examined in aqueous solution. pKa values of triazolium ions lie in the range 16.5–17.8, around 3 p''K''a units more acidic than related imidazolium ions.
Dimerisation
At one time, stable carbenes were thought to reversibly dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
ise through the so-called Wanzlick equilibrium. However, imidazol-2-ylidenes and triazol-5-ylidenes are thermodynamically stable and do not dimerise, and have been stored in solution in the absence of water and air for years. This is presumably due to the aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
nature of these carbenes, which is lost upon dimerisation. In fact imidazol-2-ylidenes are so thermodynamically stable that only in highly constrained conditions are these carbenes forced to dimerise.
Chen and Tatonmethylene bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of t ...
(–CH2–) resulted in the dicarbene dimer:
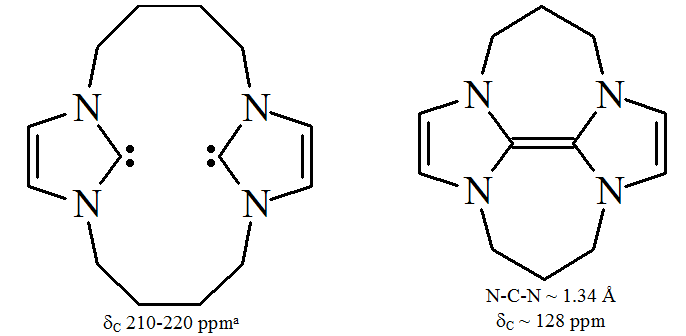 If this dimer existed as a dicarbene, the electron lone pairs on the carbenic carbon would be forced into close proximity. Presumably the resulting repulsive
If this dimer existed as a dicarbene, the electron lone pairs on the carbenic carbon would be forced into close proximity. Presumably the resulting repulsive electrostatic
Electrostatics is a branch of physics that studies electric charges at rest ( static electricity).
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word for amb ...
interactions would have a significant destabilising effect. To avoid this electronic interaction, the carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
units dimerise.
On the other hand, heteroamino carbenes (such as R2N–C–OR or R2N–C–SR) and non-aromatic carbenes such as diaminocarbenes (such as R2N–C–NR2) have been shown to dimerise,singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
dimerisation:
 Diaminocarbenes do not truly dimerise, but rather form the dimer by reaction via formamidinium salts, a protonated precursor species.
Diaminocarbenes do not truly dimerise, but rather form the dimer by reaction via formamidinium salts, a protonated precursor species.triplet state
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical r ...
carbenes, these singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
carbenes do not approach head to head ("least motion"), but rather the carbene lone pair attacks the empty carbon p-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
("non-least motion"). Carbene dimerisation can be catalyzed by both acids and metals.
Reactivity
The chemistry of stable carbenes has not been fully explored. However, Enders ''et al.''[
]
have performed a range of organic reactions involving a triazol-5-ylidene. These reactions are outlined below and may be considered as a model for other carbenes.
 These carbenes tend to behave in a
These carbenes tend to behave in a nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
fashion (e and f), performing insertion reaction
An insertion reaction is a chemical reaction where one chemical entity (a molecule or molecular fragment) interposes itself into an existing bond of typically a second chemical entity ''e.g.'':
: + \longrightarrow
The term only refers to the ...
s (b), addition reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct)..
Addition reactions are limited to chemical compounds that have multiple bonds, such as ...
s (c), +1cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
s (d, g and h), +1cycloadditions (a) as well as simple deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
s. The insertion reactions (b) probably proceed via deprotonation, resulting in the generation of a nucleophile (−XR) which can attack the generated salt giving the impression of a H–X insertion.
The reported stable isothiazole
An isothiazole, or 1,2-thiazole, is a type of organic compound containing a five-membered aromatic ring that consists of three carbon atoms, one nitrogen atom, and one sulfur atom.''Heterocyclic Chemistry'', 3rd Edition, J.A. Joule, K. Mills, and ...
carbene (2b) derived from an isothiazolium perchlorate (1)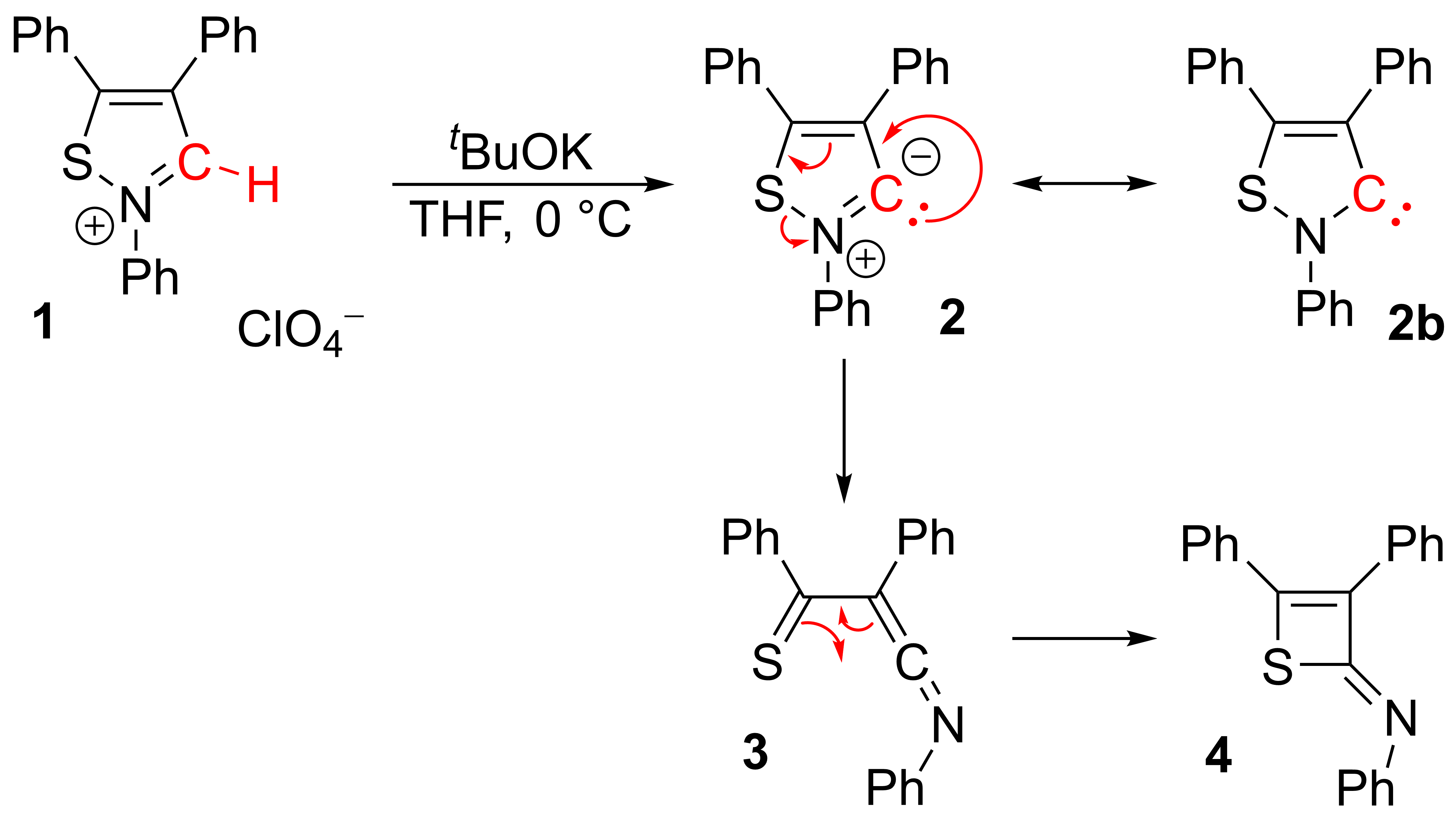
Carbene complexation
Imidazol-2-ylidenes, triazol-5-ylidenes (and less so, diaminocarbenes) have been shown to coordinate to a plethora of elements, from alkali metals
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
, main group elements, transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s and even lanthanides
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and ytt ...
and actinides
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The inform ...
. A periodic table of elements gives some idea of the complexes which have been prepared, and in many cases these have been identified by single crystal X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
.organophosphine Organophosphines are organophosphorus compounds with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphine ...
s in their coordination properties to metals. These ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
s are said to be good σ-donors through the carbenic lone pair, but poor π-acceptors due to internal ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
back-donation from the nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
atoms adjacent to the carbene centre, and so are able to coordinate to even relatively electron deficient metals. Enders [
] and Hermann[
][
] have shown that these carbenes are suitable replacements for phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands in several catalytic cycles. Whilst they have found that these ligands do not activate the metal catalyst as much as phosphine ligands they often result in more robust catalysts. Several catalytic systems have been looked into by Hermann and Enders, using catalysts containing imidazole and triazole carbene ligands, with moderate success.[ Grubbs ][
] has reported replacing a phosphine ligand (PCy3) with an imidazol-2-ylidene in the olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often create ...
catalyst RuCl2(PCy3)2CHPh, and noted increased ring closing metathesis as well as exhibiting "a remarkable air and water stability". Molecules containing two and three carbene moieties have been prepared as potential bidentate and tridentate carbene ligands.[
]
Carbenes in organometallic chemistry & catalysis
Carbenes can be stabilised as organometallic species. These transition metal carbene complex
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been r ...
es fall into two categories:
*Fischer
Fischer is a German occupational surname, meaning fisherman. The name Fischer is the fourth most common German surname. The English version is Fisher.
People with the surname A
* Abraham Fischer (1850–1913) South African public official
* A ...
carbenes in which carbenes are tethered to a metal and an electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
(usually a carbonyl),
* Schrock carbenes; in which carbenes are tethered to a metal and an electron-donating group. The reactions that such carbenes participate in are very different from those in which organic carbenes participate.
Triplet state carbene chemistry
Persistent triplet state carbenes are likely to have very similar reactivity as other non-persistent triplet state carbenes
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" m ...
.
Physical properties
 Those carbenes that have been isolated to date tend to be colorless solids with low melting points. These carbenes tend to sublime at low temperatures under high vacuum.
One of the more useful physical properties is the diagnostic chemical shift of the carbenic carbon atom in the 13C-
Those carbenes that have been isolated to date tend to be colorless solids with low melting points. These carbenes tend to sublime at low temperatures under high vacuum.
One of the more useful physical properties is the diagnostic chemical shift of the carbenic carbon atom in the 13C-NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
spectrum. Typically this peak is in the range between 200 and 300 ppm, where few other peaks appear in the 13C-NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
spectrum. An example is shown on the left for a cyclic diaminocarbene which has a carbenic peak at 238 ppm.
Upon coordination to metal centers, the 13C carbene resonance usually shifts highfield, depending on the Lewis acidity of the complex fragment. Based on this observation, Huynh ''et al.'' developed a new methodology to determine ligand donor strengths by 13C NMR analysis of ''trans''-palladium(II)-carbene complexes. The use of a 13C-labeled N-heterocyclic carbene ligand also allows for the study of mixed carbene-phosphine complexes, which undergo ''trans''-''cis''-isomerization due to the trans effect In inorganic chemistry, the trans effect is the increased lability of ligands that are trans to certain other ligands, which can thus be regarded as trans-directing ligands. It is attributed to electronic effects and it is most notable in square pla ...
.
Applications
 NHCs are widely used as ancillary ligand in organometallic chemistry. One practical application is the
NHCs are widely used as ancillary ligand in organometallic chemistry. One practical application is the ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
-based Grubbs' catalyst
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been develo ...
and NHC-Palladium Complexes for cross-coupling reactions. NHC-metal complexes, specifically Ag(I)-NHC complexes have been widely tested for their biological applications.
Preparation methods
NHCs are often strongly basic (the pKa value of the conjugate acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of an imidazol-2-ylidene was measured at ca. 24)[
] and react with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
. Clearly these reactions are performed using air-free technique Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less ...
s, avoiding compounds of even moderate acidity
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
. Although imidazolium salts are stable to nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
addition, other non-aromatic salts are not (i.e. formamidinium salts).[
]
In these cases, strong unhindered nucleophiles are avoided whether they are generated in ''situ'' or are present as an impurity in other reagents (such as LiOH in BuLi).
Several approaches have been developed in order to prepare stable carbenes, these are outlined below.
Deprotonation
Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
of carbene precursor salts with strong bases has proved a reliable route to almost all stable carbenes:
 Imidazol-2-ylidenes and dihydroimidazol-2-ylidenes, such IMes, have been prepared by the deprotonation of the respective
Imidazol-2-ylidenes and dihydroimidazol-2-ylidenes, such IMes, have been prepared by the deprotonation of the respective imidazolium
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
and dihydroimidazolium salts. The acyclic carbenes[ and the tetrahydropyrimidinyl][ based carbenes were prepared by deprotonation using strong homogeneous bases.
Several bases and reaction conditions have been employed with varying success. The degree of success has been principally dependent on the nature of the ]precursor
Precursor or Precursors may refer to:
* Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), a hypothesized genus of fossil birds that was composed of fossilized parts of u ...
being deprotonated. The major drawback with this method of preparation is the problem of isolation of the free carbene from the metals ions used in their preparation.
Metal hydride bases
One might believe that sodium or potassium hydride
Potassium hydride, KH, is the inorganic compound of potassium and hydrogen. It is an alkali metal hydride. It is a white solid, although commercial samples appear gray. It is a powerful superbase that is useful in organic synthesis. It is sold c ...
[ would be the ideal base for deprotonating these precursor salts. The hydride should react irreversibly with the loss of ]hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
to give the desired carbene, with the inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemist ...
by-products and excess hydride being removed by filtration. In practice this reaction is often too slow, requiring the addition of DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds ...
or ''t''-BuOH.[ These reagents generate soluble ]catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s, which increase the rate of reaction of this heterogeneous system, via the generation of tert-butoxide or dimsyl anion. However, these catalysts have proved ineffective for the preparation of non-imidazolium adducts as they tend to act as nucleophiles towards the precursor salts and in so doing are destroyed. The presence of hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ions as an impurity in the metal hydride could also destroy non-aromatic salts.
Deprotonation with sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
or potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
hydride in a mixture of liquid ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
/THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
at −40 °C has been reported[ for imidazole-based carbenes. Arduengo and coworkers][ managed to prepare a dihydroimidazol-2-ylidene using NaH. However, this method has not been applied to the preparation of diaminocarbenes. In some cases, ]potassium tert-butoxide
Potassium ''tert''-butoxide is the chemical compound with the formula K+(CH3)3CO−. This colourless solid is a strong base (pKa of conjugate acid around 17), which is useful in organic synthesis. It exists as a tetrameric cubane-type cluster. ...
can be employed without the addition of a metal hydride.[
]
Alkyllithiums
The use of alkyllithiums as strong bases[ has not been extensively studied, and have been unreliable for deprotonation of precursor salts. With non-aromatic salts, n-BuLi and PhLi can act as nucleophiles whilst t-BuLi can on occasion act as a source of hydride, reducing the salt with the generation of ]isobutene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Producti ...
:

Amides bases
Lithium amides like the diisopropylamide (LDA) and the ( tetramethylpiperidide (LiTMP))[ generally work well for the deprotonation of all types of salts, providing that not too much ]LiOH
Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids. They are soluble in water and slightly soluble in ethanol. Both are available commercially. While ...
is present in the ''n''-butyllithium used to make the lithium amide. Titration of lithium amide can be used to determine the amount of hydroxide in solution. The deprotonation of precursor salts with metal hexamethyldisilazides[ works very cleanly for the deprotonation of all types of salts, except for unhindered formamidinium salts, where this base can act as a nucleophile to give a triaminomethane adduct.
]
Metal-free carbene preparation
 The preparation of stable carbenes free from metal cations has been keenly sought to allow further study of the carbene species in isolation from these metals. Separating a carbene from a carbene-metal complex can be problematic due to the stability of the complex. Accordingly, it is preferable to make the carbene free from these metals in the first place. Indeed, some metal ions, rather than stabilising the carbene, have been implicated in the catalytic dimerisation of unhindered examples.
Shown right is an X-ray structure showing a complex between a diaminocarbene and potassium HMDS. This complex was formed when excess KHMDS was used as a strong base to deprotonate the formamidinium salt. Removing lithium ions resulting from deprotonation with reagents such as lithium diisopropylamide (LDA) can be especially problematic. Potassium and sodium salt by-products tend to precipitate from solution and can be removed. Lithium ions may be chemically removed by binding to species such as
The preparation of stable carbenes free from metal cations has been keenly sought to allow further study of the carbene species in isolation from these metals. Separating a carbene from a carbene-metal complex can be problematic due to the stability of the complex. Accordingly, it is preferable to make the carbene free from these metals in the first place. Indeed, some metal ions, rather than stabilising the carbene, have been implicated in the catalytic dimerisation of unhindered examples.
Shown right is an X-ray structure showing a complex between a diaminocarbene and potassium HMDS. This complex was formed when excess KHMDS was used as a strong base to deprotonate the formamidinium salt. Removing lithium ions resulting from deprotonation with reagents such as lithium diisopropylamide (LDA) can be especially problematic. Potassium and sodium salt by-products tend to precipitate from solution and can be removed. Lithium ions may be chemically removed by binding to species such as cryptand
In chemistry, cryptands are a family of synthetic, bicyclic and polycyclic, multidentate ligands for a variety of cations. The Nobel Prize for Chemistry in 1987 was given to Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen for their ...
s or crown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Impo ...
s.
Metal free carbenes have been prepared in several ways as outlined below:
Dechalcogenation
Another approach of preparing carbenes has relied on the desulfurisation of thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea a ...
s with potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
in THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
.[ A contributing factor to the success of this reaction is that the byproduct, potassium sulfide, is insoluble in the solvent. The elevated temperatures suggest that this method is not suitable for the preparation of unstable dimerising carbenes. A single example of the ]deoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to orga ...
of a urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
with a fluorene derived carbene to give the tetramethyldiaminocarbene and fluorenone has also been reported:
 The desulfurisation of thioureas with molten
The desulfurisation of thioureas with molten potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
to give imidazol-2-ylidenes or diaminocarbenes has not been widely used. The method was used to prepare dihydroimidazole carbenes.[
]
Vacuum pyrolysis
Vacuum pyrolysis, with the removal of neutral volatile byproducts i.e. methanol or chloroform, has been used to prepare dihydroimidazole and triazole based carbenes. Historically the removal of chloroform by vacuum pyrolysis of adducts A was used by Wanzlick[ in his early attempts to prepare dihydroimidazol-2-ylidenes but this method is not widely used. The Enders laboratory][ has used vacuum pyrolysis of adduct B to generate a triazol-5-ylidene.
]
Bis(trimethylsilyl)mercury
Bis(trimethylsilyl)mercury (CH3)3Si-Hg-Si(CH3)3 reacts with chloro-iminium
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with al ...
and chloro- amidinium salts to give a metal-free carbene and elemental mercury.
Photochemical decomposition
Persistent triplet state carbenes have been prepared by photochemical
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400–7 ...
decomposition of a diazomethane product via the expulsion of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
gas, at a wavelength of 300 nm in benzene.
Purification
 Stable carbenes are very reactive, and so the minimum amount of handling is desirable using
Stable carbenes are very reactive, and so the minimum amount of handling is desirable using air-free technique Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less ...
s. However, provided rigorously dry, relatively non-acidic and air-free materials are used, stable carbenes are reasonably robust to handling ''per se''. By way of example, a stable carbene prepared from potassium hydride can be filtered through a dry celite pad to remove excess KH (and resulting salts) from the reaction. On a relatively small scale, a suspension containing a stable carbene in solution can be allowed to settle and the supernatant solution pushed through a dried membrane syringe filter. Stable carbenes are readily soluble in non-polar solvents such as hexane, and so typically recrystallisation of stable carbenes can be difficult, due to the unavailability of suitable non-acidic polar solvents. Air-free sublimation as shown right can be an effective method of purification, although temperatures below 60 °C under high vacuum are preferable as these carbenes are relatively volatile and also could begin to decompose at these higher temperatures. Indeed, sublimation in some cases can give single crystals suitable for X-ray analysis. However, strong complexation to metal ions like lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
will in most cases prevent sublimation.
References
Further reading
Reviews on persistent carbenes:
*.
Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents, (Chapter 4, Hideo Tomioka (triplet state); Chapter 5 (singlet state), Roger W. Alder) - ed. Guy BertrandReactive Intermediate Chemistry By Robert A. Moss, Matthew Platz, Maitland Jones (Chapter 8, Stable Singlet Carbenes, Guy Bertrand)
*R. W. Alder, in 'Diaminocarbenes: exploring structure and reactivity', ed. G. Bertrand, New York, 2002
*
For a review on the physico-chemical properties (electronics, sterics, ...) of N-heterocyclic carbenes:
*
{{good article
Functional groups
Carbenes
Organometallic chemistry
Organic compounds
 A persistent carbene (also known as stable carbene) is a type of
A persistent carbene (also known as stable carbene) is a type of  This exchange was proposed to proceed via intermediacy of a thiazol-2-ylidene. In 2012 the isolation of the so-called ''Breslow intermediate'' was reported.
In 1960, Hans-Werner Wanzlick and coworkers conjectured that carbenes derived from dihydroimidazol-2-ylidene were produced by vacuum pyrolysis of the corresponding 2-trichloromethyl dihydroimidazole compounds with the loss of chloroform.
They conjectured that the carbene existed in equilibrium with its
This exchange was proposed to proceed via intermediacy of a thiazol-2-ylidene. In 2012 the isolation of the so-called ''Breslow intermediate'' was reported.
In 1960, Hans-Werner Wanzlick and coworkers conjectured that carbenes derived from dihydroimidazol-2-ylidene were produced by vacuum pyrolysis of the corresponding 2-trichloromethyl dihydroimidazole compounds with the loss of chloroform.
They conjectured that the carbene existed in equilibrium with its  In 1988, Guy Bertrand and others isolated a phosphinocarbene. These species can be represented as either a λ3-phosphinocarbene or λ5- phosphaacetylene:
In 1988, Guy Bertrand and others isolated a phosphinocarbene. These species can be represented as either a λ3-phosphinocarbene or λ5- phosphaacetylene:
 These compounds were called "push-pull carbenes" in reference to the contrasting electron affinities of the phosphorus and silicon atoms. They exhibit both carbenic and alkynic reactivity. An X-ray structure of this molecule has not been obtained and at the time of publication some doubt remained as to their exact carbenic nature.
In 1991, Arduengo and coworkers crystallized a diaminocarbene by
These compounds were called "push-pull carbenes" in reference to the contrasting electron affinities of the phosphorus and silicon atoms. They exhibit both carbenic and alkynic reactivity. An X-ray structure of this molecule has not been obtained and at the time of publication some doubt remained as to their exact carbenic nature.
In 1991, Arduengo and coworkers crystallized a diaminocarbene by  This carbene, the forerunner of a large family of carbenes with the imidazol-2-ylidene core, is indefinitely stable at room temperature in the absence of oxygen and moisture. It melts at 240–241 °C without decomposition. The 13C NMR spectrum shows a signal at 211 ppm for the carbenic atom. The
This carbene, the forerunner of a large family of carbenes with the imidazol-2-ylidene core, is indefinitely stable at room temperature in the absence of oxygen and moisture. It melts at 240–241 °C without decomposition. The 13C NMR spectrum shows a signal at 211 ppm for the carbenic atom. The 
 The first acyclic persistent carbene was reported in 1996, thus showing that a cyclic backbone was not necessary for their stability. Unlike the cyclic derivatives, the acyclic carbenes are flexible with respect to rotation of the bonds to the carbenic atom. By measuring the barrier to rotation of these bonds, the extent of their
The first acyclic persistent carbene was reported in 1996, thus showing that a cyclic backbone was not necessary for their stability. Unlike the cyclic derivatives, the acyclic carbenes are flexible with respect to rotation of the bonds to the carbenic atom. By measuring the barrier to rotation of these bonds, the extent of their  Unlike NHCs, these carbenes are not thermodynamically stable but tend to dimerize.
In bis(diisopropylamino)cyclopropenylidene, which is stable at room temperature, the carbene atom is connected to two carbon atoms, in a three-member ring that retains the aromaticity and geometry of the cyclopropenylidene ring. This example demonstrated that the presence of heteroatoms next to the carbene is not necessary for stability, either.
Unlike NHCs, these carbenes are not thermodynamically stable but tend to dimerize.
In bis(diisopropylamino)cyclopropenylidene, which is stable at room temperature, the carbene atom is connected to two carbon atoms, in a three-member ring that retains the aromaticity and geometry of the cyclopropenylidene ring. This example demonstrated that the presence of heteroatoms next to the carbene is not necessary for stability, either.
 For the
For the  In particular, substitution of two
In particular, substitution of two  The triazol-5-ylidenes based on the
The triazol-5-ylidenes based on the  Triazole-based carbenes are thermodynamically stable and have diagnostic 13C NMR chemical shift values between 210 and 220 ppm for the carbenic carbon. The X-ray structure of the triphenyl substituted carbene above shows an N–C–N bond angle of around 101°. The 5-methoxytriazole precursor to this carbene was made by the treatment of a triazolium salt with sodium methoxide, which attacks as a nucleophile. This may indicate that these carbenes are less aromatic than imidazol-2-ylidenes, as the imidazolium precursors do not react with nucleophiles due to the resultant loss of aromaticity.
Triazole-based carbenes are thermodynamically stable and have diagnostic 13C NMR chemical shift values between 210 and 220 ppm for the carbenic carbon. The X-ray structure of the triphenyl substituted carbene above shows an N–C–N bond angle of around 101°. The 5-methoxytriazole precursor to this carbene was made by the treatment of a triazolium salt with sodium methoxide, which attacks as a nucleophile. This may indicate that these carbenes are less aromatic than imidazol-2-ylidenes, as the imidazolium precursors do not react with nucleophiles due to the resultant loss of aromaticity.
 Unlike the aromatic imidazol-2-ylidenes or triazol-5-ylidenes, these carbenes appear not to be thermodynamically stable, as shown by the dimerisation of some unhindered cyclic and acyclic examples. Studies suggest that these carbenes dimerise via acid catalysed dimerisation (as in the Wanzlick equilibrium).
Diaminocarbenes have diagnostic 13C NMR chemical shift values between 230 and 270 ppm for the carbenic atom. The X-ray structure of dihydroimidazole-2-ylidene shows a N–C–N bond angle of about 106°, whilst the angle of the acyclic carbene is 121°, both greater than those seen for imidazol-2-ylidenes.
Unlike the aromatic imidazol-2-ylidenes or triazol-5-ylidenes, these carbenes appear not to be thermodynamically stable, as shown by the dimerisation of some unhindered cyclic and acyclic examples. Studies suggest that these carbenes dimerise via acid catalysed dimerisation (as in the Wanzlick equilibrium).
Diaminocarbenes have diagnostic 13C NMR chemical shift values between 230 and 270 ppm for the carbenic atom. The X-ray structure of dihydroimidazole-2-ylidene shows a N–C–N bond angle of about 106°, whilst the angle of the acyclic carbene is 121°, both greater than those seen for imidazol-2-ylidenes.
 In particular, the formal substitution of sulfur for one of the nitrogens in imidazole would yield the aromatic heterocyclic compound
In particular, the formal substitution of sulfur for one of the nitrogens in imidazole would yield the aromatic heterocyclic compound 
 Exposure to oxygen (a triplet diradical) converts this carbene to the corresponding
Exposure to oxygen (a triplet diradical) converts this carbene to the corresponding  However, further work showed that diaminocarbenes will deprotonate the DMSO solvent, with the resulting anion reacting with the resulting amidinium salt.
However, further work showed that diaminocarbenes will deprotonate the DMSO solvent, with the resulting anion reacting with the resulting amidinium salt.
 Reaction of imidazol-2-ylidenes with 1-bromohexane gave 90% of the 2-substituted adduct, with only 10% of the corresponding
Reaction of imidazol-2-ylidenes with 1-bromohexane gave 90% of the 2-substituted adduct, with only 10% of the corresponding  If this dimer existed as a dicarbene, the electron lone pairs on the carbenic carbon would be forced into close proximity. Presumably the resulting repulsive
If this dimer existed as a dicarbene, the electron lone pairs on the carbenic carbon would be forced into close proximity. Presumably the resulting repulsive  Diaminocarbenes do not truly dimerise, but rather form the dimer by reaction via formamidinium salts, a protonated precursor species. Accordingly, this reaction can be acid catalysed. This reaction occurs because unlike imidazolium based carbenes, there is no loss of aromaticity in protonation of the carbene.
Unlike the dimerisation of
Diaminocarbenes do not truly dimerise, but rather form the dimer by reaction via formamidinium salts, a protonated precursor species. Accordingly, this reaction can be acid catalysed. This reaction occurs because unlike imidazolium based carbenes, there is no loss of aromaticity in protonation of the carbene.
Unlike the dimerisation of  These carbenes tend to behave in a
These carbenes tend to behave in a 
 Those carbenes that have been isolated to date tend to be colorless solids with low melting points. These carbenes tend to sublime at low temperatures under high vacuum.
One of the more useful physical properties is the diagnostic chemical shift of the carbenic carbon atom in the 13C-
Those carbenes that have been isolated to date tend to be colorless solids with low melting points. These carbenes tend to sublime at low temperatures under high vacuum.
One of the more useful physical properties is the diagnostic chemical shift of the carbenic carbon atom in the 13C- NHCs are widely used as ancillary ligand in organometallic chemistry. One practical application is the
NHCs are widely used as ancillary ligand in organometallic chemistry. One practical application is the  Imidazol-2-ylidenes and dihydroimidazol-2-ylidenes, such IMes, have been prepared by the deprotonation of the respective
Imidazol-2-ylidenes and dihydroimidazol-2-ylidenes, such IMes, have been prepared by the deprotonation of the respective 
 The preparation of stable carbenes free from metal cations has been keenly sought to allow further study of the carbene species in isolation from these metals. Separating a carbene from a carbene-metal complex can be problematic due to the stability of the complex. Accordingly, it is preferable to make the carbene free from these metals in the first place. Indeed, some metal ions, rather than stabilising the carbene, have been implicated in the catalytic dimerisation of unhindered examples.
Shown right is an X-ray structure showing a complex between a diaminocarbene and potassium HMDS. This complex was formed when excess KHMDS was used as a strong base to deprotonate the formamidinium salt. Removing lithium ions resulting from deprotonation with reagents such as lithium diisopropylamide (LDA) can be especially problematic. Potassium and sodium salt by-products tend to precipitate from solution and can be removed. Lithium ions may be chemically removed by binding to species such as
The preparation of stable carbenes free from metal cations has been keenly sought to allow further study of the carbene species in isolation from these metals. Separating a carbene from a carbene-metal complex can be problematic due to the stability of the complex. Accordingly, it is preferable to make the carbene free from these metals in the first place. Indeed, some metal ions, rather than stabilising the carbene, have been implicated in the catalytic dimerisation of unhindered examples.
Shown right is an X-ray structure showing a complex between a diaminocarbene and potassium HMDS. This complex was formed when excess KHMDS was used as a strong base to deprotonate the formamidinium salt. Removing lithium ions resulting from deprotonation with reagents such as lithium diisopropylamide (LDA) can be especially problematic. Potassium and sodium salt by-products tend to precipitate from solution and can be removed. Lithium ions may be chemically removed by binding to species such as  The desulfurisation of thioureas with molten
The desulfurisation of thioureas with molten 
 Stable carbenes are very reactive, and so the minimum amount of handling is desirable using
Stable carbenes are very reactive, and so the minimum amount of handling is desirable using