|
Potassium Bis(trimethylsilyl)amide
Potassium bis(trimethylsilyl)amide (commonly abbreviated as KHMDS, Potassium(K) HexaMethylDiSilazide) or potassium hexamethyldisilazane is the chemical compound with the formula ((CH3)3Si)2NK. It is a strong, non-nucleophilic base with an approximate pKa of 26 (compare to lithium diisopropylamide, at 36). Structure In the solid state, the unsolvated compound is dimeric, with two potassium and two nitrogen atoms forming a square. This compound is soluble in hydrocarbon solvents and conducts electricity poorly in solution and in the melt. This is attributed to very strong ion pairing.{{cite journal , doi = 10.1021/ic00333a029 , journal = Inorg. Chem. , title = Ion pairing in is(trimethylsilyl)amidootassium: The x-ray crystal structure of unsolvated N(SiMe3)2 , year = 1990 , last1 = Tesh , first1 = Kris F. , last2 = Hanusa , first2 = Timothy P. , last3 = Huffman , first3 = John C. , volume = 29 , issue = 8 , pages = 1584–1586 See also * Metal bis(trimethylsilyl)amid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Bis(trimethylsilyl)amide
Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula . It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species. Preparation LiHMDS is commercially available, but it can also be prepared by the deprotonation of bis(trimethylsilyl)amine with ''n''-butyllithium. This reaction can be performed ''in situ''. : Once formed, the compound can be purified by sublimation or distillation. Reactions and applications As a base LiHMDS is often used in organic chemistry as a strong non-nucleophilic base. Its conjugate acid has a p''K''a of ~26, making it is less basic than other lithium bases, such as LDA (p''K''a of conjugate acid ~36), but it is more sterically hindered and hence less nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Bis(trimethylsilyl)amide
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula . This species, usually called NaHMDS (sodium hexamethyldisilazide), is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups. NaHMDS is quickly destroyed by water to form sodium hydroxide and bis(trimethylsilyl)amine. Structure Although the Na–N bond is polar covalent as a solid, when dissolved in nonpolar solvents this compound is trimeric, consisting of a central ring. Applications in synthesis NaHMDS is used as a strong base in organic synthesis. Typical reactions: *To deprotonate ketones and esters to generate enolate derivatives. *Generate carbenes by dehydrohalogenation of halocarbons. These carbene reagents add to alkenes to give su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Diisopropylamide
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula . It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group. Preparation and structure LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of tetrahydrofuran and diisopropylamine with ''n''-butyllithium. When dissociated, the diisopropylamide anion can become protonated to form diisopropylamine. Diisopropylamine has a p''K''a value of 36. Therefore, its conjugate base is suitable for the deprotonation of compounds with greater acidity, importantly, such weakly acidic compounds (carbon acids) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Pair
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each other, as ion pairs, ion triplets, etc. Ion pairs are also classified according to the nature of the interaction as contact, solvent-shared or solvent-separated. The most important factor to determine the extent of ion association is the dielectric constant of the solvent. Ion associates have been characterized by means of vibrational spectroscopy, as introduced by Niels Bjerrum, and dielectric-loss spectroscopy. Classification of ion pairs ''Ion pairs'' are formed when a cation and anion, which are present in a solution of an ionizable substance, come together to form a discrete chemical species. There are three distinct types of ''ion pairs'', depending on the extent of solvation of the two ions. For example, magnesium sulphate ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
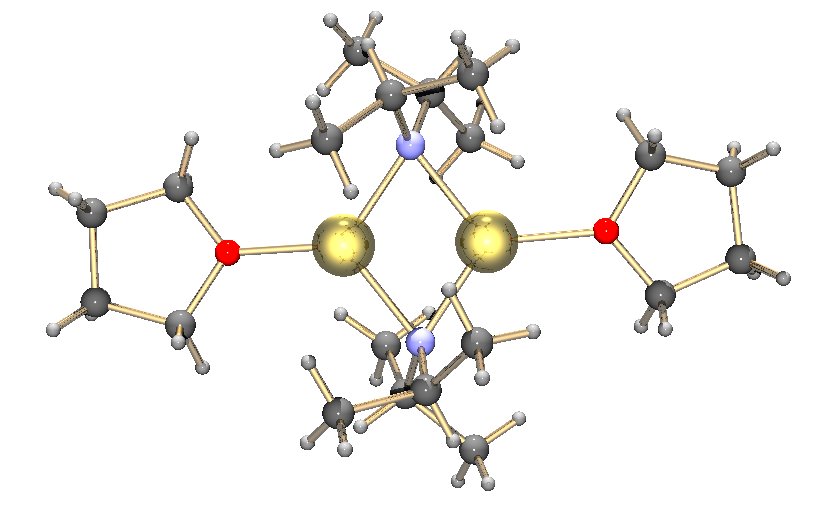
Metal Bis(trimethylsilyl)amides
Metal bis(trimethylsilyl)amides (often abbreviated as metal silylamides) are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides. Due to the bulky hydrocarbon backbone metal bis(trimethylsilyl)amide complexes have low lattice energies and are lipophilic . For this reason, they are soluble in a range of nonpolar organic solvents, in contrast to simple metal halides, which only dissolve in reactive solvents. These steric bulky complexes are molecular, consisting of mono-, di-, and tetramers. Having a built-in base, these compounds conveniently react with even weakly protic reagents. The class of ligands and pioneering studies on their coordination compounds were described by Bürger and Wannagat. The ligands are often denoted ''hmds'' (e.g. M(N(SiMe3)2)3 = M(hmds)3) in reference to the hexamethyldisilazane from which they are prepared. General methods of preparation Apart from group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Compounds
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

2.png)
