Iodoform test on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, the haloform reaction is a 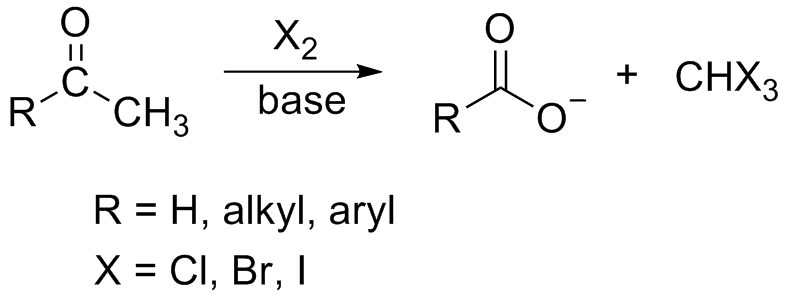
Br2 + 2 OH- -> Br- + BrO- + H2O
If a secondary alcohol is present, it is oxidized to a ketone by the hypohalite:
If a methyl ketone is present, it reacts with the hypohalite in a three-step process:
1. Under basic conditions, the ketone undergoes keto-enol tautomerisation. The enolate undergoes electrophilic attack by the hypohalite (containing a halogen with a formal +1 charge).
: 2. When the α(alpha) position has been exhaustively halogenated, the molecule undergoes a
2. When the α(alpha) position has been exhaustively halogenated, the molecule undergoes a 
chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
in which a haloform
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane () are replaced by halogen atoms. Many trihalomethanes find uses in industry as solvents or refrigerants. THMs are also environmenta ...
(, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (, where R can be either a hydrogen atom, an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
or an aryl group
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
), in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl group
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s () or to produce chloroform (), bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluorofor ...
(), or iodoform
Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula C H I3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older ch ...
(). Note that fluoroform
Trifluoromethane or fluoroform is the chemical compound with the formula CHF3. It is one of the " haloforms", a class of compounds with the formula CHX3 (X = halogen) with C3v symmetry. Fluoroform is used in diverse applications in organic s ...
() can't be prepared in this way.

Mechanism
In the first step, the halogen dis-proportionates in the presence ofhydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
to give the halide and hypohalite.
:nucleophilic acyl substitution
Nucleophilic acyl substitution describe a class of substitution reactions involving nucleophiles and acyl compounds. In this type of reaction, a nucleophile – such as an alcohol, amine, or enolate – displaces the leaving group of an acyl deriv ...
by hydroxide, with being the leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
stabilized by three electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
s. In the third step the anion abstracts a proton from either the solvent or the carboxylic acid formed in the previous step, and forms the haloform. At least in some cases (chloral hydrate
Chloral hydrate is a geminal diol with the formula . It is a colorless solid. It has limited use as a sedative and hypnotic pharmaceutical drug. It is also a useful laboratory chemical reagent and precursor. It is derived from chloral (trichl ...
) the reaction may stop and the intermediate product isolated if conditions are acidic and hypohalite is used.
:Scope
Substrates are broadly limited to methyl ketones and secondary alcohols oxidizable to methyl ketones, such as isopropanol. The only primary alcohol andaldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
to undergo this reaction are ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
and acetaldehyde, respectively. 1,3-Diketones such as acetylacetone also give the haloform reaction. β-ketoacids such as acetoacetic acid
Acetoacetic acid (also acetoacetate and diacetic acid) is the organic compound with the formula CHCOCHCOOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite sta ...
will also give the test upon heating. Acetyl chloride and acetamide don't give this test. The halogen used may be chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
, bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
, iodine or sodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium s ...
. Fluoroform
Trifluoromethane or fluoroform is the chemical compound with the formula CHF3. It is one of the " haloforms", a class of compounds with the formula CHX3 (X = halogen) with C3v symmetry. Fluoroform is used in diverse applications in organic s ...
(CHF3) cannot be prepared by this method as it would require the presence of the highly unstable hypofluorite ion. However ketones with the structure RCOCF3 do cleave upon treatment with base to produce fluoroform; this is equivalent to the second and third steps in the process shown above.
Applications
Laboratory scale
This reaction forms the basis of the iodoform test which was commonly used in history as achemical test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group.
Purposes
Chemical testing might have a variety of purposes, such as to:
* Determin ...
to determine the presence of a methyl ketone, or a secondary alcohol oxidizable to a methyl ketone. When iodine and sodium hydroxide are used as the reagents a positive reaction gives iodoform
Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula C H I3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older ch ...
, which is a solid at room temperature and tends to precipitate out of solution causing a distinctive cloudiness.
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
, this reaction may be used to convert a terminal methyl ketone into the analogous carboxylic acid.
Industrially
It was formerly used to produce iodoform, bromoform, and even chloroform industrially.As a by-product of water chlorination
Water chlorination
Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spr ...
can result in the formation of haloforms if the water contains suitable reactive impurities (e.g. humic acid). There is a concern that such reactions may lead to the presence of carcinogenic compounds in drinking water.
History
The haloform reaction is one of the oldest organic reactions known. In 1822,Georges-Simon Serullas Georges-Simon Serullas (2 November 1774 in Poncin – 25 May 1832 in Paris) was a professor of pharmacy notable for being the first to publish a work on Iodoform, an early antiseptic and disinfectant.
Biography
He was a professor and head pharm ...
added potassium metal to a solution of iodine in ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
and water to form potassium formate and iodoform, called in the language of that time ''hydroiodide of carbon''. In 1832, Justus von Liebig reported the reaction of chloral
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless oily liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate ...
with calcium hydroxide to form chloroform and calcium formate. The reaction was rediscovered by Adolf Lieben in 1870.See:
*
* The iodoform test is also called the Lieben haloform reaction. A review of the haloform reaction with a history section was published in 1934.
References
{{Reflist, 2 Organic redox reactions Carbon-heteroatom bond forming reactions Halogenation reactions