Heusler alloy on:
[Wikipedia]
[Google]
[Amazon]
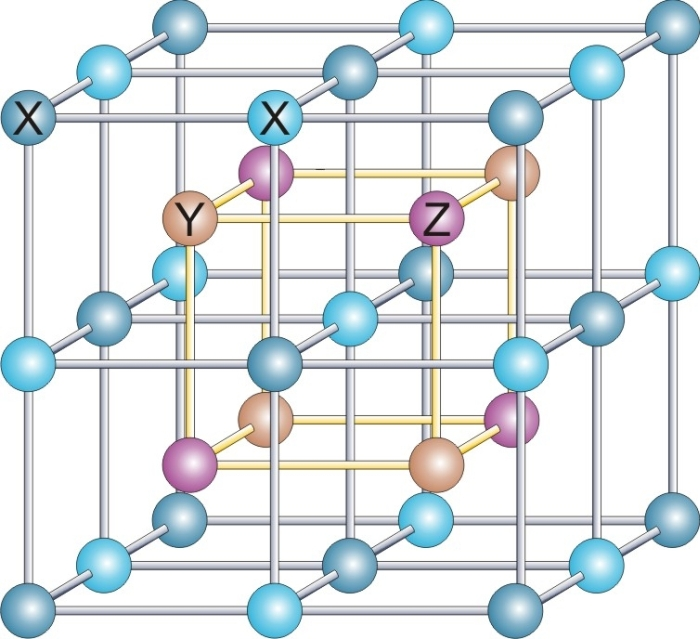 Heusler compounds are
Heusler compounds are
 The half-Heusler compounds have distinctive properties and high tunability which makes the class very promising as thermoelectric materials. A study has predicted that there can be as many as 481 stable half-Heusler compounds using high-throughput ab initio calculation combine with machine learning techniques. The particular half-Heusler compounds of interest as
The half-Heusler compounds have distinctive properties and high tunability which makes the class very promising as thermoelectric materials. A study has predicted that there can be as many as 481 stable half-Heusler compounds using high-throughput ab initio calculation combine with machine learning techniques. The particular half-Heusler compounds of interest as
National Pollutant Inventory – Copper and compounds fact sheet
Copper alloys Intermetallics Magnetic alloys Ferromagnetic materials Spintronics Crystal structure types
 Heusler compounds are
Heusler compounds are magnetic
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particl ...
intermetallic
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
s with face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s and Z is in the p-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
. The term derives from the name of German
German(s) may refer to:
* Germany (of or related to)
**Germania (historical use)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizens of Germany, see also German nationality law
**Ge ...
mining engineer
Mining in the engineering discipline is the extraction of minerals from underneath, open pit, above or on the ground. Mining engineering is associated with many other disciplines, such as mineral processing, exploration, excavation, geology, a ...
and chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
Friedrich Heusler, who studied such a compound (Cu2MnAl) in 1903. Many of these compounds exhibit properties relevant to spintronics
Spintronics (a portmanteau meaning spin transport electronics), also known as spin electronics, is the study of the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid- ...
, such as magnetoresistance
Magnetoresistance is the tendency of a material (often ferromagnetic) to change the value of its electrical resistance in an externally-applied magnetic field. There are a variety of effects that can be called magnetoresistance. Some occur in bu ...
, variations of the Hall effect
The Hall effect is the production of a voltage difference (the Hall voltage) across an electrical conductor that is transverse to an electric current in the conductor and to an applied magnetic field perpendicular to the current. It was dis ...
, ferro-, antiferro-, and ferrimagnetism, half- and semimetallicity, semiconductivity with spin filter ability, superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
, topological band structure and are actively studied as Thermoelectric materials
Thermoelectric materials show the thermoelectric effect in a strong or convenient form.
The ''thermoelectric effect'' refers to phenomena by which either a temperature difference creates an electric potential or an electric current creates a t ...
. Their magnetism results from a double-exchange mechanism The double-exchange mechanism is a type of a magnetic exchange that may arise between ions in different oxidation states. First proposed by Clarence Zener, this theory predicts the relative ease with which an electron may be exchanged between two s ...
between neighboring magnetic ions. Manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
, which sits at the body centers of the cubic structure, was the magnetic ion in the first Heusler compound discovered. (See the Bethe–Slater curve
The Bethe–Slater curve is a heuristic explanation for why certain metals are ferromagnetic and others are antiferromagnetic. It assumes a Heisenberg model of magnetism, and explains the differences in exchange energy of transition metals as du ...
for details of why this happens.)
Styles of writing chemical formula
Depending on the field of literature being surveyed, one might encounter the same compound referred to with different chemical formulas. An example of the most common difference is X2YZ versus XY2Z, where the reference to the two transition metals X and Y in the compound are swapped. The traditional convention X2YZ arises from the interpretation of Heuslers as intermetallics and used predominantly in literature studying magnetic applications of Heuslers compounds. The XY2Z convention on the other hand is used mostly in thermoelectric materials and transparent conducting applications literature where semiconducting Heuslers (most half-Heuslers are semiconductors) are used. This convention, in which the left-most element on the periodic table comes first, uses the Zintl interpretation of semiconducting compounds where the chemical formula XY2Z is written in order of increasing electronegativity. In well-known compounds such as Fe2VAl which were historically thought of as metallic (semi-metallic) but were more recently shown to be small-gap semiconductors one might find both styles being used. In the present article semiconducting compounds might sometimes be mentioned in the XY2Z style."Off-Stoichiometric" Heuslers
Although traditionally thought to form at compositions XYZ and X2YZ, studies published after 2015 are discovering and reliably predicting Heusler compounds at atypical compositions such as XY0.8Z and X1.5YZ. Besides these ternary compositions, quaternary Heusler compositions called the double Half-Heusler X2YY'Z2 (e.g. Ti2FeNiSb2) and triple Half-Heusler X2X'Y3Z3 (for e.g. Mg2VNi3Sb3) have also been discovered. Additionally, Li-based quaternary LiXYZ have been predicted from calculations. These `off-stoichiometric' (the off-stoichiometry here refers to their deviation from the well-known XYZ and X2YZ compositions. Although, in principle, these are compounds with new stoichiometries) Heuslers are mostly semiconductors, and in the low temperature T = 0 K limit stabilize at stoichiometries unquiely identifiable by their valence balanced configuration. The stable compositions and corresponding electrical properties however, can be quite sensitive to temperature. The order-disorder transition temperatures in these off-stoichiometric compounds can also often occur below room-temperatures. Large amounts of defects at the atomic scale in off-stoichiometric Heuslers helps them achieve very low thermal conductivities and make them favorable for thermoelectric applications. X1.5YZ semiconducting composition is stabilized by the transition metal X playing a dual role (electron donor as well as acceptor) in the structure.Magnetic properties
The early studies Full-Heusler compound Cu2MnAl has the following properties. Its magnetism varies considerably with heat treatment and composition. It has a room-temperature saturation induction of around 8,000 gauss, which exceeds that of the elementnickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
(around 6100 gauss) but is smaller than that of iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
(around 21500 gauss). For early studies see. In 1934, Bradley and Rogers showed that the room-temperature ferromagnetic phase was a fully ordered structure of the L21 Strukturbericht type. This has a primitive cubic lattice of copper atoms with alternate cells body-centered by manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
and aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
. The lattice parameter is 5.95 Å. The molten alloy has a solidus
Solidus (Latin for "solid") may refer to:
* Solidus (coin), a Roman coin of nearly solid gold
* Solidus (punctuation), or slash, a punctuation mark
* Solidus (chemistry), the line on a phase diagram below which a substance is completely solid
* ...
temperature of about 910 °C. As it is cooled below this temperature, it transforms into disordered, solid, body-centered cubic beta-phase. Below 750 °C, a B2 ordered lattice forms with a primitive cubic copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
lattice, which is body-centered by a disordered manganese-aluminium sublattice. Cooling below 610 °C causes further ordering of the manganese and aluminium sub-lattice to the L21 form. In non-stoichiometric alloys, the temperatures of ordering decrease, and the range of anealing temperatures, where the alloy does not form microprecipitates, becomes smaller than for the stoichiometric material.
Oxley found a value of 357 °C for the Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cur ...
, below which the compound becomes ferromagnetic. Neutron diffraction and other techniques have shown that a magnetic moment of around 3.7 Bohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
The Bohr magneton, in SI units is defined as
\mu_\m ...
s resides almost solely on the manganese atoms. As these atoms are 4.2 Å apart, the exchange interaction, which aligns the spins, is likely indirect and is mediated through conduction electrons or the aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
and copper atoms.
Electron microscopy
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
studies demonstrated that thermal antiphase boundaries (APBs) form during cooling through the ordering temperatures, as ordered domains nucleate at different centers within the crystal lattice and are often out of step with each other where they meet. The anti-phase domains grow as the alloy is annealed. There are two types of APBs corresponding to the B2 and L21 types of ordering. APBs also form between dislocations if the alloy is deformed. At the APB the manganese atoms will be closer than in the bulk of the alloy and, for non-stoichiometric alloys with an excess of copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
(e.g. Cu2.2MnAl0.8), an antiferromagnetic layer forms on every thermal APB. These antiferromagnetic layers completely supersede the normal magnetic domain
A magnetic domain is a region within a magnetic material in which the magnetization is in a uniform direction. This means that the individual magnetic moments of the atoms are aligned with one another and they point in the same direction. When c ...
structure and stay with the APBs if they are grown by annealing the alloy. This significantly modifies the magnetic properties of the non-stoichiometric alloy relative to the stoichiometric alloy which has a normal domain structure. Presumably this phenomenon is related to the fact that pure manganese is an antiferromagnet
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. ...
although it is not clear why the effect is not observed in the stoichiometric alloy. Similar effects occur at APBs in the ferromagnetic alloy MnAl at its stoichiometric composition.
Some Heusler compounds also exhibit properties of materials known as ferromagnetic shape-memory alloys. These are generally composed of nickel, manganese and gallium and can change their length by up to 10% in a magnetic field.
Mechanical properties
Understanding the mechanical properties of Heusler compounds is paramount for temperature-sensitive applications (e.g. thermoelectrics) for which some sub-classes of Heusler compounds are used. However, experimental studies are rarely encountered in literature. In fact, the commercialization of these compounds is limited by the material’s ability to undergo intense, repetitivethermal cycling
Thermal analysis is a branch of materials science where the properties of materials are studied as they change with temperature. Several methods are commonly used – these are distinguished from one another by the property which is measured:
* ...
and resist cracking from vibrations. An appropriate measure for crack resistance is the material’s toughness
In materials science and metallurgy, toughness is the ability of a material to absorb energy and plastically deform without fracturing.mechanical strength
The field of strength of materials, also called mechanics of materials, typically refers to various methods of calculating the stresses and strains in structural members, such as beams, columns, and shafts. The methods employed to predict the re ...
. In this section, we highlight existing experimental and computational studies on the mechanical properties of Heusler alloys. Note that the mechanical properties of such a compositionally-diverse class of materials is expectedly dependent on the chemical composition of the alloys themselves, and therefore trends in mechanical properties are difficult to identify without a case-by-case study.
The elastic modulus
An elastic modulus (also known as modulus of elasticity) is the unit of measurement of an object's or substance's resistance to being deformed elastically (i.e., non-permanently) when a stress is applied to it. The elastic modulus of an object is ...
values of half-Heusler alloys range from 83 to 207 GPa, whereas the bulk modulus
The bulk modulus (K or B) of a substance is a measure of how resistant to compression the substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume.
Other moduli descri ...
spans a tighter range from 100 GPa in HfNiSn to 130 GPa in TiCoSb. A collection of various density functional theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
(DFT) calculations show that half-Heusler compounds are predicted to have a lower elastic, shear, and bulk modulus than in quaternary-, full-, and inverse-Hausler alloys. DFT also predicts a decrease in elastic modulus with temperature in Ni2XAl (X=Sc, Ti, V), as well as an increase in stiffness
Stiffness is the extent to which an object resists deformation in response to an applied force.
The complementary concept is flexibility or pliability: the more flexible an object is, the less stiff it is.
Calculations
The stiffness, k, of a ...
with pressure. The decrease in modulus with respect to temperature is also observed in TiNiSn, ZrNiSn, and HfNiSn, where ZrNiSn has the highest modulus and Hf has the lowest. This phenomenon can be explained by the fact that the elastic modulus decreases with increasing interatomic separation: as temperature increases, the atomic vibrations also increase, resulting in a larger equilibrium interatomic separation.
The mechanical strength is also rarely studied in Heusler compounds. One study has shown that, in off-stoichiometric Ni2MnIn, the material reaches a peak strength of 475 MPa at 773 K, which drastically reduces to below 200 MPa at 973 K. In another study, a polycrystalline
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains.
Bacillite is a type of crystallite. It is rodlike with parallel longulites.
Stru ...
Heusler alloy composed of the Ni-Mn-Sn ternary composition space was found to possess a peak compressive strength of about 2000 MPa with plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adapta ...
deformation up to 5%. However, the addition of Indium
Indium is a chemical element with the symbol In and atomic number 49. Indium is the softest metal that is not an alkali metal. It is a silvery-white metal that resembles tin in appearance. It is a post-transition metal that makes up 0.21 parts ...
to the Ni-Mn-Sn ternary alloy not only increases the porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measur ...
of the samples, but it also reduces the compressive strength to 500 MPa. It is unclear from the study what percentage of the porosity increase from the Indium addition reduces the strength. Note that this is opposite to the outcome expected from solid solution strengthening
In metallurgy, solid solution strengthening is a type of alloying that can be used to improve the strength of a pure metal. The technique works by adding atoms of one element (the alloying element) to the crystalline lattice of another element ...
, where adding Indium to the ternary system slows dislocation movement through dislocation-solute interaction and subsequently increases the material's strength.
The fracture toughness
In materials science, fracture toughness is the critical stress intensity factor of a sharp crack where propagation of the crack suddenly becomes rapid and unlimited. A component's thickness affects the constraint conditions at the tip of a ...
can also be tuned with composition modifications. For example, the average toughness of Ti1−x(Zr, Hf)xNiSn ranges from 1.86 MPa m1/2 to 2.16 MPa m1/2, increasing with Zr/Hf content. The preparation of samples may affect the measured fracture toughness however, as elaborated by O’Connor et al. In their study, samples of Ti0.5Hf0.5Co0.5Ir0.5Sb1−xSnx were prepared using three different methods: a high-temperature solid state reaction, high-energy ball milling, and a combination of both. The study found higher fracture toughness in samples prepared without a high-energy ball milling step of 2.7 MPa m1/2 to 4.1 MPa m1/2, as opposed to samples that were prepared with ball milling of 2.2 MPa m1/2 to 3.0 MPa m1/2. Fracture toughness is sensitive to inclusions and existing cracks in the material, so it is as expected dependent the sample preparation.
Half-Heusler Thermoelectrics
 The half-Heusler compounds have distinctive properties and high tunability which makes the class very promising as thermoelectric materials. A study has predicted that there can be as many as 481 stable half-Heusler compounds using high-throughput ab initio calculation combine with machine learning techniques. The particular half-Heusler compounds of interest as
The half-Heusler compounds have distinctive properties and high tunability which makes the class very promising as thermoelectric materials. A study has predicted that there can be as many as 481 stable half-Heusler compounds using high-throughput ab initio calculation combine with machine learning techniques. The particular half-Heusler compounds of interest as thermoelectric materials
Thermoelectric materials show the thermoelectric effect in a strong or convenient form.
The ''thermoelectric effect'' refers to phenomena by which either a temperature difference creates an electric potential or an electric current creates a t ...
(space group ) are the semiconducting ternary compounds with a general formula XYZ where X is a more electropositive transition metal (such as Ti or Zr), Y is a less electropositive
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
transition metal (such Ni or Co), and Z is heavy main group element (such as Sn or Sb). This flexible range of element selection allows many different combinations to form a half-Heusler phase and enables a diverse range of material properties.
Half-Heusler thermoelectric materials have distinct advantages over many other thermoelectric materials; low toxicity, inexpensive element, robust mechanical properties, and high thermal stability make half-Heusler thermoelectrics an excellent option for mid-high temperature application. However, the high thermal conductivity, which is intrinsic to highly symmetric HH structure, has made HH thermoelectric generally less efficient than other classes of TE materials. Many studies have focused on improving HH thermoelectric by reducing the lattice thermal conductivity and zT > 1 has been repeatedly recorded.
Half-Metallic Ferromagnetic Heusler
Half-metallic ferromagnets exhibit a metallic behavior in one spin channel and an insulating behavior in the other spin channel. The first example of Heusler Half-metallic ferromagnets was first investigated by de Groot et al., with the case of NiMnSb. Half-metallicity leads to the full polarization of the conducting electrons. Half metallic ferromagnets are therefore promising forSpintronics
Spintronics (a portmanteau meaning spin transport electronics), also known as spin electronics, is the study of the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid- ...
applications.
List of notable Heusler compounds
*Cu2MnAl, Cu2MnIn, Cu2MnSn *Ni2MnAl, Ni2MnIn, Ni2MnSn, Ni2MnSb, Ni2MnGa *Co2MnAl, Co2MnSi, Co2MnGa, Co2MnGe, Co2NiGa *Pd2MnAl, Pd2MnIn, Pd2MnSn, Pd2MnSb *Co2FeSi, Co2FeAl *Fe2VAl *Mn2VGa, Co2FeGe *Co2CrxFe1−xX(X=Al, Si)See also
* YbBiPtReferences
Further reading
*G. Sauthoff: Intermetallics, Wiley-VCH, Weinheim 1995, S. 83 u. 90. * *{{cite journal , doi=10.1080/00107516908204800 , title=Heusler alloys , journal=Contemporary Physics , volume=10 , issue=6 , pages=559–577 , year=1969 , last1=Webster , first1=Peter J , bibcode=1969ConPh..10..559WExternal links
National Pollutant Inventory – Copper and compounds fact sheet
Copper alloys Intermetallics Magnetic alloys Ferromagnetic materials Spintronics Crystal structure types