GABAA receptor on:
[Wikipedia]
[Google]
[Amazon]
 The GABAA receptor (GABAAR) is an
The GABAA receptor (GABAAR) is an
 Structural understanding of the GABAA receptor was initially based on homology models, obtained using crystal structures of homologous proteins like Acetylcholine binding protein (AChBP) and nicotinic acetylcholine (nACh) receptors as templates. The much sought structure of a GABAA receptor was finally resolved, with the disclosure of the crystal structure of human β3 homopentameric GABAA receptor.
Whilst this was a major development, the majority of GABAA receptors are heteromeric and the structure did not provide any details of the benzodiazepine binding site. This was finally elucidated in 2018 by the publication of a high resolution cryo-EM structure of rat α1β1γ2S receptor and human α1β2γ2 receptor bound with GABA and the neutral benzodiazepine flumazenil.
GABAA receptors are
Structural understanding of the GABAA receptor was initially based on homology models, obtained using crystal structures of homologous proteins like Acetylcholine binding protein (AChBP) and nicotinic acetylcholine (nACh) receptors as templates. The much sought structure of a GABAA receptor was finally resolved, with the disclosure of the crystal structure of human β3 homopentameric GABAA receptor.
Whilst this was a major development, the majority of GABAA receptors are heteromeric and the structure did not provide any details of the benzodiazepine binding site. This was finally elucidated in 2018 by the publication of a high resolution cryo-EM structure of rat α1β1γ2S receptor and human α1β2γ2 receptor bound with GABA and the neutral benzodiazepine flumazenil.
GABAA receptors are
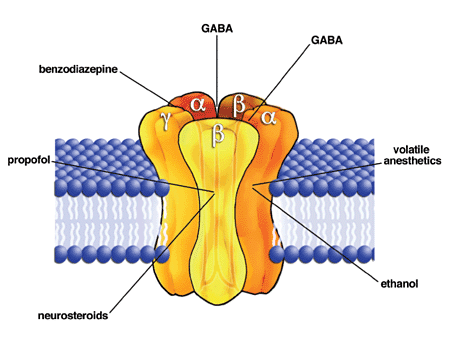 A number of
A number of
 The GABAA receptor (GABAAR) is an
The GABAA receptor (GABAAR) is an ionotropic receptor
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in res ...
and ligand-gated ion channel
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in res ...
. Its endogenous ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
in the central nervous system
The central nervous system (CNS) is the part of the nervous system consisting primarily of the brain and spinal cord. The CNS is so named because the brain integrates the received information and coordinates and influences the activity of all par ...
. Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−). Depending on the membrane potential and the ionic concentration difference, this can result in ionic fluxes across the pore. If the membrane potential is higher than the equilibrium potential In a biological membrane, the reversal potential is the membrane potential at which the direction of ionic current reverses. At the reversal potential, there is no net flow of ions from one side of the membrane to the other. For channels that are pe ...
(also known as the reversal potential) for chloride ions, when the receptor is activated Cl− will flow into the cell. This causes an inhibitory effect on neurotransmission
Neurotransmission (Latin: ''transmissio'' "passage, crossing" from ''transmittere'' "send, let through") is the process by which signaling molecules called neurotransmitters are released by the axon terminal of a neuron (the presynaptic neuron), ...
by diminishing the chance of a successful action potential
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, ...
occurring at the postsynaptic cell. The reversal potential of the GABAA-mediated inhibitory postsynaptic potential
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential.Purves et al. Neuroscience. 4th ed. Sunderland (MA): Sinauer Associates, Incorporated; 2008. ...
(IPSP) in normal solution is −70 mV, contrasting the GABAB IPSP (-100 mV).
The active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) a ...
of the GABAA receptor is the binding site for GABA and several drugs such as muscimol
Muscimol (also known as agarin or pantherine) is one of the principal psychoactive constituents of '' Amanita muscaria'' and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptors and displa ...
, gaboxadol
Gaboxadol, also known as 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP), is a conformationally constrained derivative of the alkaloid muscimol that was first synthesized in 1977 by the Danish chemist Povl Krogsgaard-Larsen. In the early 19 ...
, and bicuculline. The protein also contains a number of different allosteric binding sites which modulate the activity of the receptor indirectly. These allosteric sites are the targets of various other drugs, including the benzodiazepines, nonbenzodiazepine
Nonbenzodiazepines (), sometimes referred to colloquially as Z-drugs (as many of their names begin with the letter "z"), are a class of psychoactive drugs that are very benzodiazepine-like in nature. They are used in the treatment of sleep proble ...
s, neuroactive steroids, barbiturates, alcohol (ethanol), inhaled anaesthetics, kavalactones
Kavalactones are a class of lactone compounds found in kava roots and Alpinia zerumbet (Shell ginger). Kavalactones are under research for potential to have various psychotropic effects, including anxiolytic and sedative/ hypnotic activities.
En ...
, cicutoxin, and picrotoxin
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Gree ...
, among others.
GABAA receptors occur in all organisms that have a nervous system. To a limited extent the receptors can be found in non-neuronal tissues. Due to their wide distribution within the nervous system of mammals they play a role in virtually all brain functions.
Target for benzodiazepines
Theionotropic
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in res ...
GABAA receptor protein complex is also the molecular target of the benzodiazepine class of tranquilizer drugs. Benzodiazepines do not bind to the same receptor ''site'' on the protein complex as the endogenous ligand GABA (whose binding site is located between α- and β-subunits), but bind to distinct benzodiazepine binding sites situated at the interface between the α- and γ-subunits of α- and γ-subunit containing GABAA receptors. While the majority of GABAA receptors (those containing α1-, α2-, α3-, or α5-subunits) are benzodiazepine sensitive, there exists a minority of GABAA receptors (α4- or α6-subunit containing) which are insensitive to classical 1,4-benzodiazepines, but instead are sensitive to other classes of GABAergic drugs such as neurosteroids and alcohol. In addition peripheral benzodiazepine receptors exist which are not associated with GABAA receptors. As a result, the IUPHAR
The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ...
has recommended that the terms "''BZ receptor''", "''GABA/BZ receptor''" and "''omega receptor''" no longer be used and that the term "''benzodiazepine receptor''" be replaced with "benzodiazepine site".
In order for GABAA receptors to be sensitive to the action of benzodiazepines they need to contain an α and a γ subunit, between which the benzodiazepine binds. Once bound, the benzodiazepine locks the GABAA receptor into a conformation where the neurotransmitter GABA has much higher affinity for the GABAA receptor, increasing the frequency of opening of the associated chloride ion channel and hyperpolarising the membrane. This potentiates the inhibitory effect of the available GABA leading to sedative and anxiolytic effects.
Different benzodiazepines have different affinities for GABAA receptors made up of different collection of subunits, and this means that their pharmacological profile varies with subtype selectivity. For instance, benzodiazepine receptor ligands with high activity at the α1 and/or α5 tend to be more associated with sedation, ataxia
Ataxia is a neurological sign consisting of lack of voluntary coordination of muscle movements that can include gait abnormality, speech changes, and abnormalities in eye movements. Ataxia is a clinical manifestation indicating dysfunction of ...
and amnesia
Amnesia is a deficit in memory caused by brain damage or disease,Gazzaniga, M., Ivry, R., & Mangun, G. (2009) Cognitive Neuroscience: The biology of the mind. New York: W.W. Norton & Company. but it can also be caused temporarily by the use ...
, whereas those with higher activity at GABAA receptors containing α2 and/or α3 subunits generally have greater anxiolytic
An anxiolytic (; also antipanic or antianxiety agent) is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxi ...
activity. Anticonvulsant
Anticonvulsants (also known as antiepileptic drugs or recently as antiseizure drugs) are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of b ...
effects can be produced by agonists acting at any of the GABAA subtypes, but current research in this area is focused mainly on producing α2-selective agonists as anticonvulsants which lack the side effects of older drugs such as sedation and amnesia.
The binding site for benzodiazepines is distinct from the binding site for barbiturates and GABA on the GABAA receptor, and also produces different effects on binding, with the benzodiazepines increasing the frequency of the chloride channel opening, while barbiturates increase the duration of chloride channel opening when GABA is bound. Since these are separate modulatory effects, they can both take place at the same time, and so the combination of benzodiazepines with barbiturates is strongly synergistic, and can be dangerous if dosage is not strictly controlled.
Also note that some GABAA agonists such as muscimol
Muscimol (also known as agarin or pantherine) is one of the principal psychoactive constituents of '' Amanita muscaria'' and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptors and displa ...
and gaboxadol
Gaboxadol, also known as 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP), is a conformationally constrained derivative of the alkaloid muscimol that was first synthesized in 1977 by the Danish chemist Povl Krogsgaard-Larsen. In the early 19 ...
do bind to the same site on the GABAA receptor complex as GABA itself, and consequently produce effects which are similar but not identical to those of positive allosteric modulators like benzodiazepines.
Structure and function
 Structural understanding of the GABAA receptor was initially based on homology models, obtained using crystal structures of homologous proteins like Acetylcholine binding protein (AChBP) and nicotinic acetylcholine (nACh) receptors as templates. The much sought structure of a GABAA receptor was finally resolved, with the disclosure of the crystal structure of human β3 homopentameric GABAA receptor.
Whilst this was a major development, the majority of GABAA receptors are heteromeric and the structure did not provide any details of the benzodiazepine binding site. This was finally elucidated in 2018 by the publication of a high resolution cryo-EM structure of rat α1β1γ2S receptor and human α1β2γ2 receptor bound with GABA and the neutral benzodiazepine flumazenil.
GABAA receptors are
Structural understanding of the GABAA receptor was initially based on homology models, obtained using crystal structures of homologous proteins like Acetylcholine binding protein (AChBP) and nicotinic acetylcholine (nACh) receptors as templates. The much sought structure of a GABAA receptor was finally resolved, with the disclosure of the crystal structure of human β3 homopentameric GABAA receptor.
Whilst this was a major development, the majority of GABAA receptors are heteromeric and the structure did not provide any details of the benzodiazepine binding site. This was finally elucidated in 2018 by the publication of a high resolution cryo-EM structure of rat α1β1γ2S receptor and human α1β2γ2 receptor bound with GABA and the neutral benzodiazepine flumazenil.
GABAA receptors are pentamer
A pentamer is an entity composed of five sub-units.
In chemistry, it applies to molecules made of five monomers.
In biochemistry, it applies to macromolecules, in particular to pentameric proteins, made of five proteic sub-units.
In microbiol ...
ic transmembrane receptor
Cell surface receptors (membrane receptors, transmembrane receptors) are receptor (biochemistry), receptors that are embedded in the cell membrane, plasma membrane of cell (biology), cells. They act in cell signaling by receiving (binding to) ex ...
s which consist of five subunits arranged around a central pore
Pore may refer to:
Biology Animal biology and microbiology
* Sweat pore, an anatomical structure of the skin of humans (and other mammals) used for secretion of sweat
* Hair follicle, an anatomical structure of the skin of humans (and other m ...
. Each subunit comprises four transmembrane domains with both the N- and C-terminus located extracellularly. The receptor sits in the membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. B ...
of its neuron
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. N ...
, usually localized at a synapse
In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or to the target effector cell.
Synapses are essential to the transmission of nervous impulses from ...
, postsynaptically. However, some isoforms may be found extrasynaptically. When vesicles
Vesicle may refer to:
; In cellular biology or chemistry
* Vesicle (biology and chemistry), a supramolecular assembly of lipid molecules, like a cell membrane
* Synaptic vesicle
; In human embryology
* Vesicle (embryology), bulge-like features o ...
of GABA are released presynaptically and activate the GABA receptors at the synapse, this is known as phasic inhibition. However, the GABA escaping from the synaptic cleft can activate receptors on presynaptic terminals or at neighbouring synapses on the same or adjacent neurons (a phenomenon termed ‘spillover’) in addition to the constant, low GABA concentrations in the extracellular space results in persistent activation of the GABAA receptors known as tonic inhibition.
The ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
GABA is the endogenous compound that causes this receptor to open; once bound to GABA, the protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
receptor changes conformation within the membrane, opening the pore in order to allow chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s (Cl−) and, to a lesser extent, bicarbonate ions (HCO3−) to pass down their electrochemical gradient
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a membrane. The gradient consists of two parts, the chemical gradient, or difference in solute concentration across a membrane, and ...
. The binding site to GABA is about 80Å away from the narrowest part of the ion channel. Recent computational studies have suggested an allosteric mechanism whereby GABA binding leads to ion channel opening. Because the reversal potential In a biological membrane, the reversal potential is the membrane potential at which the direction of ionic current reverses. At the reversal potential, there is no net flow of ions from one side of the membrane to the other. For channels that are pe ...
for chloride in most mature neurons is close to or more negative than the resting membrane potential, activation of GABAA receptors tends to stabilize or hyperpolarise the resting potential, and can make it more difficult for excitatory neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neuro ...
s to depolarize the neuron and generate an action potential
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, ...
. The net effect therefore typically inhibitory, reducing the activity of the neuron, although depolarizing currents have been observed in response to GABA in immature neurons in early development. This effect during development is due to a modified Cl− gradient wherein the anions leave the cells through the GABAA receptors, since their intracellular chlorine concentration is higher than the extracellular. The difference in extracellular chlorine anion concentration is presumed to be due to the higher activity of chloride transporters, such as NKCC1
The Na-K-Cl cotransporter (NKCC) is a protein that aids in the secondary active transport of sodium, potassium, and chloride into cells. In humans there are two isoforms of this membrane transport protein, NKCC1 and NKCC2, encoded by two differe ...
, transporting chloride into cells which are present early in development, whereas, for instance, KCC2
Potassium-chloride transporter member 5 (aka: KCC2 and SLC12A5) is a neuron-specific chloride potassium symporter responsible for establishing the chloride ion gradient in neurons through the maintenance of low intracellular chloride concentratio ...
transports chloride out cells and is the dominant factor in establishing the chloride gradient later in development. These depolarization events have shown to be key in neuronal development. In the mature neuron, the GABAA channel opens quickly and thus contributes to the early part of the inhibitory post-synaptic potential
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a Chemical synapse, postsynaptic neuron less likely to generate an action potential.Purves et al. Neuroscience. 4th ed. Sunderland (MA): Sinauer Associates, Inc ...
(IPSP).
The endogenous ligand that binds to the benzodiazepine site is inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring (also known as a ribofuranose) via a β-N9- glycosidic bond. It was discovered in 1965 in analysis of RNA transferase.
Inosine is commonly found in tRNAs and is ...
.
Subunits
GABAA receptors are members of the large pentameric ligand gated ion channel (previously referred to as "''Cys''-loop" receptors) super-family of evolutionarily related and structurally similarligand-gated ion channel
Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in res ...
s that also includes nicotinic acetylcholine receptor
Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral ner ...
s, glycine receptor
The glycine receptor (abbreviated as GlyR or GLR) is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory ...
s, and the 5HT3 receptor. There are numerous subunit isoform
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene or gene family and are the result of genetic differences. While many perform the same or similar biological roles, some iso ...
s for the GABAA receptor, which determine the receptor's agonist affinity, chance of opening, conductance, and other properties.
In humans, the units are as follows:
* six types of α subunits (GABRA1
Gamma-aminobutyric acid receptor subunit alpha-1 is a protein that in humans is encoded by the ''GABRA1'' gene.
GABA is the major inhibitory neurotransmitter in the mammalian brain where it acts at GABA-A receptors, which are ligand-gated chlor ...
, GABRA2
Gamma-aminobutyric acid receptor subunit alpha-2 is a protein in humans that is encoded by the ''GABRA2'' gene.
GABRA2 is an alpha subunit that is part of GABA-A receptors, which are ligand-gated chloride channels and are activated by the majo ...
, GABRA3
Gamma-aminobutyric acid receptor subunit alpha-3 is a protein that in humans is encoded by the ''GABRA3'' gene.
Function
GABA is the major inhibitory neurotransmitter in the mammalian brain where it acts at GABAA receptors, which are ligand- ...
, GABRA4
Gamma-aminobutyric acid receptor subunit alpha-4 is a protein that in humans is encoded by the ''GABRA4'' gene.
GABA is the major inhibitory neurotransmitter in the mammalian brain where it acts at GABA-A receptors, which are ligand-gated chlorid ...
, GABRA5
Gamma-aminobutyric acid (GABA) A receptor, alpha 5, also known as GABRA5, is a protein which in humans is encoded by the ''GABRA5'' gene.
Function
GABA is the major inhibitory neurotransmitter in the mammalian brain where it acts at GABAA recept ...
, GABRA6
Gamma-aminobutyric acid receptor subunit alpha-6 is a protein that in humans is encoded by the ''GABRA6'' gene.
GABA is the major inhibitory neurotransmitter in the mammalian brain where it acts at GABA-A receptors, which are ligand-gated chlorid ...
)
* three βs (GABRB1
Gamma-aminobutyric acid receptor subunit beta-1 is a protein that in humans is encoded by the ''GABRB1'' gene.
Function
The gamma-aminobutyric acid A receptor ( GABAA receptor) is a multisubunit chloride channel that mediates the fastest inhibi ...
, GABRB2
The GABAA beta-2 subunit is a protein that in humans is encoded by the GABRB2 gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or '' ...
, GABRB3
Gamma-aminobutyric acid receptor subunit beta-3 is a protein that in humans is encoded by the ''GABRB3'' gene. It is located within the 15q12 region in the human genome and spans 250kb. This gene includes 10 exons within its coding region. Due to a ...
)
* three γs (GABRG1
Gamma-aminobutyric acid receptor subunit gamma-1 is a protein that in humans is encoded by the ''GABRG1'' gene. The protein encoded by this gene is a subunit of the GABAA receptor.
Variants of this gene may be associated with alcohol dependenc ...
, GABRG2
Gamma-aminobutyric acid receptor subunit gamma-2 is a protein that in humans is encoded by the ''GABRG2'' gene.
Function
Gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain, mediates neuronal inhibition by binding ...
, GABRG3
GABAA receptor-γ3, also known as GABRG3, is a protein which in humans is encoded by the ''GABRG3'' gene.
Function
GABRG3 is a subunit of the GABAA receptor for the neurotransmitter gamma-Aminobutyric acid ( GABA).
Association with alcoholism ...
)
* as well as a δ (GABRD
Gamma-aminobutyric acid receptor subunit delta is a protein that in humans is encoded by the ''GABRD'' gene. In the mammalian brain, the delta (δ) subunit forms specific GABAA receptor subtypes by co-assembly leading to δ subunit containing GABA ...
), an ε (GABRE
Gamma-aminobutyric acid receptor subunit epsilon is a protein that in humans is encoded by the ''GABRE'' gene.
The product of this gene belongs to the ligand-gated ionic channel (TC 1.A.9) family. It encodes the gamma-aminobutyric acid (GABA) A r ...
), a π (GABRP
Gamma-aminobutyric acid receptor subunit pi is a protein that in humans is encoded by the ''GABRP'' gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meanin ...
), and a θ (GABRQ
Gamma-aminobutyric acid receptor subunit theta is a protein that in humans is encoded by the ''GABRQ'' gene. The protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. P ...
)
There are three ρ units (GABRR1
Gamma-aminobutyric acid receptor subunit rho-1 is a protein that in humans is encoded by the ''GABRR1'' gene.
GABA is the major inhibitory neurotransmitter in the mammalian brain where it acts at GABA receptors, which are ligand-gated chloride cha ...
, GABRR2, GABRR3
Gamma-aminobutyric acid receptor subunit rho-3 is a protein that in humans is encoded by the ''GABRR3'' gene. The protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Pr ...
); however, these do not coassemble with the classical GABAA units listed above, but rather homooligomerize to form GABAA-ρ receptors (formerly classified as GABAC receptors but now this nomenclature
Nomenclature (, ) is a system of names or terms, or the rules for forming these terms in a particular field of arts or sciences. The principles of naming vary from the relatively informal naming conventions, conventions of everyday speech to the i ...
has been deprecated).
Combinatorial Arrays
Given the large number of GABAA receptors, a great diversity of final pentameric receptor subtypes is possible. Methods to produce cell-based laboratory access to a greater number of possible GABAA receptor subunit combinations allow teasing apart of the contribution of specific receptor subtypes and their physiological and pathophysiological function and role in the CNS and in disease.Distribution
GABAA receptors are responsible for most of the physiological activities of GABA in the central nervous system, and the receptor subtypes vary significantly. Subunit composition can vary widely between regions and subtypes may be associated with specific functions. The minimal requirement to produce a GABA-gated ion channel is the inclusion of an α and a β subunit. The most common GABAA receptor is a pentamer comprising two α's, two β's, and a γ (α2β2γ). In neurons themselves, the type of GABAA receptor subunits and their densities can vary betweencell bodies
The soma (pl. ''somata'' or ''somas''), perikaryon (pl. ''perikarya''), neurocyton, or cell body is the bulbous, non-process portion of a neuron or other brain cell type, containing the cell nucleus. The word 'soma' comes from the Greek '' σῶ� ...
and dendrites
Dendrites (from Greek δένδρον ''déndron'', "tree"), also dendrons, are branched protoplasmic extensions of a nerve cell that propagate the electrochemical stimulation received from other neural cells to the cell body, or soma, of the ...
. GABAA receptors can also be found in other tissues, including leydig cells
Leydig cells, also known as interstitial cells of the testes and interstitial cells of Leydig, are found adjacent to the seminiferous tubules in the testicle and produce testosterone in the presence of luteinizing hormone (LH). They are polyhedra ...
, placenta
The placenta is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate mater ...
, immune cells
White blood cells, also called leukocytes or leucocytes, are the cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. All white blood cells are produced and derived from mult ...
, liver
The liver is a major Organ (anatomy), organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the Protein biosynthesis, synthesis of proteins and biochemicals necessary for ...
, bone growth plates and several other endocrine tissues. Subunit expression varies between 'normal' tissue and malignancies
Malignancy () is the tendency of a medical condition to become progressively worse.
Malignancy is most familiar as a characterization of cancer. A ''malignant'' tumor contrasts with a non-cancerous ''benign'' tumor in that a malignancy is not s ...
, as GABAA receptors can influence cell proliferation.
Ligands
 A number of
A number of ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
have been found to bind to various sites on the GABAA receptor complex and modulate it besides GABA itself. A ligand can possess one or more properties of the following types. Unfortunately the literature often does not distinguish these types properly.
Types
* Orthosteric agonists andantagonists
An antagonist is a character in a story who is presented as the chief foe of the protagonist.
Etymology
The English word antagonist comes from the Greek ἀνταγωνιστής – ''antagonistēs'', "opponent, competitor, villain, enemy, riv ...
: bind to the main receptor site (the site where GABA normally binds, also referred to as the "active" or "orthosteric" site). Agonists activate the receptor, resulting in increased Cl− conductance. Antagonists, though they have no effect on their own, compete with GABA for binding and thereby inhibit its action, resulting in decreased Cl− conductance.
* First order allosteric modulators: bind to allosteric sites on the receptor complex and affect it either in a positive (PAM), negative (NAM) or neutral/silent (SAM) manner, causing increased or decreased efficiency of the main site and therefore an indirect increase or decrease in Cl− conductance. SAMs do not affect the conductance, but occupy the binding site.
* Second order modulators: bind to an allosteric site on the receptor complex and modulate the effect of first order modulators.
* Open channel blockers: prolong ligand-receptor occupancy, activation kinetics and Cl ion flux in a subunit configuration-dependent and sensitization-state dependent manner.
* Non-competitive channel blockers: bind to or near the central pore of the receptor complex and directly block Cl− conductance through the ion channel.
Examples
* Orthosteric agonists: GABA,gaboxadol
Gaboxadol, also known as 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (THIP), is a conformationally constrained derivative of the alkaloid muscimol that was first synthesized in 1977 by the Danish chemist Povl Krogsgaard-Larsen. In the early 19 ...
, isoguvacine
Isoguvacine is a GABAA receptor agonist used in scientific research.
See also
* Gaboxadol
* Muscimol
Muscimol (also known as agarin or pantherine) is one of the principal psychoactive constituents of '' Amanita muscaria'' and related specie ...
, muscimol
Muscimol (also known as agarin or pantherine) is one of the principal psychoactive constituents of '' Amanita muscaria'' and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptors and displa ...
, progabide
Progabide (INN; trade name Gabrene, Sanofi-Aventis) is an analogue and prodrug of γ-aminobutyric acid (GABA) used in the treatment of epilepsy. Via conversion into GABA, progabide behaves as an agonist of the GABAA, GABAB, and GABAA-ρ re ...
, beta-alanine, taurine, piperidine-4-sulfonic acid (partial agonist).
* Orthosteric antagonists: bicuculline, gabazine
Gabazine (SR-95531) is a drug that acts as an Receptor antagonist, antagonist at GABAA receptor, GABAA Receptor (biochemistry), receptors. It is used in scientific research and has no role in medicine, as it would be expected to produce convulsio ...
.
* Positive allosteric modulators: barbiturates, benzodiazepines, certain carbamates
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
(ex. carisoprodol, meprobamate
Meprobamate—marketed as Miltown by Wallace Laboratories and Equanil by Wyeth, among others—is a carbamate derivative used as an anxiolytic drug. It was the best-selling minor tranquilizer for a time, but has largely been replaced by the be ...
, lorbamate), Honokiol
Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus ''Magnolia''. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methyl ...
, Magnolol
Magnolol is an organic compound that is classified as lignan. It is a bioactive compound found in the bark of the Houpu magnolia (''Magnolia officinalis'') or in '' M. grandiflora''. The compound exists at the level of a few percent in the bark o ...
, Baicalin
As baicalin is a flavone glycoside, it is a flavonoid. It is the glucuronide of baicalein.
Natural occurrences
Baicalin is found in several species in the genus ''Scutellaria'', including ''Scutellaria baicalensis'', and '' Scutellaria lateri ...
, Baicelin, thienodiazepine
A thienodiazepine is a heterocyclic compound containing a diazepine ring fused to a thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extens ...
s, alcohol (ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
), etomidate
Etomidate (USAN, INN, BAN; marketed as Amidate) is a short-acting intravenous anaesthetic agent used for the induction of general anaesthesia and sedation for short procedures such as reduction of dislocated joints, tracheal intubation, cardi ...
, glutethimide, kavalactone
Kavalactones are a class of lactone compounds found in kava roots and Alpinia zerumbet (Shell ginger). Kavalactones are under research for potential to have various psychotropic effects, including anxiolytic and sedative/ hypnotic activities.
En ...
s, meprobamate
Meprobamate—marketed as Miltown by Wallace Laboratories and Equanil by Wyeth, among others—is a carbamate derivative used as an anxiolytic drug. It was the best-selling minor tranquilizer for a time, but has largely been replaced by the be ...
, quinazolinone
Quinazolinone is a heterocyclic chemical compound, a quinazoline with a carbonyl group in the C4N2 ring. Two isomers are possible: 2-quinazolinone and 4-quinazolinone, with the 4-isomer being the more common. These compounds are of interest in me ...
s (ex. methaqualone
Methaqualone is a hypnotic sedative. It was sold under the brand names Quaalude ( ) and Sopor among others, which contained 300 mg of methaqualone, and sold as a combination drug under the brand name Mandrax, which contained 250 mg me ...
, etaqualone
Etaqualone (Aolan, Athinazone, Ethinazone) is a quinazolinone-class GABAergic and is an analogue of methaqualone that was developed in the 1960s and marketed mainly in France and some other European countries. It has sedative, hypnotic, muscle ...
, diproqualone
Diproqualone is a quinazolinone class GABAergic and is an analogue of methaqualone developed in the late 1950s by a team at Nogentaise de Produits Chimique. It was marketed primarily in France and some other European countries. It has sedative ...
), neuroactive steroids,(a) ; (b) ; (c); (d) ; (e) ; (f) ; (g) ; (h) ; (i) ; (j) ; (k) niacin
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variet ...
/niacinamide
Niacinamide or Nicotinamide (NAM) is a form of vitamin B3 found in food and used as a dietary supplement and medication. As a supplement, it is used by mouth to prevent and treat pellagra (niacin deficiency). While nicotinic acid (niacin) may b ...
, nonbenzodiazepine
Nonbenzodiazepines (), sometimes referred to colloquially as Z-drugs (as many of their names begin with the letter "z"), are a class of psychoactive drugs that are very benzodiazepine-like in nature. They are used in the treatment of sleep proble ...
s (ex. zolpidem
Zolpidem, sold under the brand name Ambien, among others, is a medication primarily used for the short-term treatment of sleeping problems. Guidelines recommend that it be used only after cognitive behavioral therapy for insomnia and behavior ...
, eszopiclone
Eszopiclone, sold under the brand-name Lunesta among others such as Night Calm in Egypt, is a medication used in the treatment of insomnia. Evidence supports slight to moderate benefit up to six months. It is taken orally.
Common side effects i ...
), propofol, stiripentol
Stiripentol, sold under the brand name Diacomit, is an anticonvulsant medication used for the treatment of Dravet syndrome - a serious genetic brain disorder.
The most common side effects include loss of appetite, weight loss, insomnia (difficu ...
, theanine
Theanine , also known as L-γ-glutamylethylamide and ''N''5-ethyl-L-glutamine, is an amino acid analogue of the proteinogenic amino acids L-glutamate and L-glutamine and is found primarily in particular plant and fungal species. It was disco ...
, valerenic acid
Valerenic acid is a sesquiterpenoid constituent of the essential oil of the valerian plant.
Valerian is used as a herbal sedative which may be helpful in the treatment of insomnia. Valerenic acid may be at least partly responsible for valeri ...
, volatile/inhaled anesthetic
An anesthetic (American English) or anaesthetic (British English; see spelling differences) is a drug used to induce anesthesia — in other words, to result in a temporary loss of sensation or awareness. They may be divided into two ...
s, lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lant ...
, and riluzole.
* Negative allosteric modulators: flumazenil
Flumazenil (also known as flumazepil, code name Ro 15-1788) is a selective GABAA receptor antagonist administered via injection, otic insertion, or intranasally. Therapeutically, it acts as both an antagonist and antidote to benzodiazepines ( ...
, Ro15-4513
Ro15-4513 ''(IUPAC: Ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo-1,4-benzodiazepine-3-carboxylate)'' is a weak partial inverse agonist of the benzodiazepine class of drugs, developed by Hoffmann–La Roche in the 1980s. It acts as a invers ...
, sarmazenil
Sarmazenil (Ro15-3505) is a drug from the benzodiazepine family. It acts as a partial inverse agonist of benzodiazepine receptors, meaning that it causes the opposite effects to most benzodiazepine drugs, and instead acts as an anxiogenic and con ...
, Pregnenolone sulfate
Pregnenolone sulfate (PS, PREGS) is an endogenous excitatory neurosteroid that is synthesized from pregnenolone. It is known to have cognitive and memory-enhancing, antidepressant, anxiogenic, and proconvulsant effects.
Biological activity
Pr ...
, amentoflavone
Amentoflavone is a biflavonoid (''bis''-apigenin coupled at 8 and 3' positions, or 3′,8′′-biapigenin) constituent of a number of plants including ''Ginkgo biloba'', ''Chamaecyparis obtusa'' (hinoki), '' Hypericum perforatum'' (St ...
, and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
.
* Inverse allosteric agonists: beta-carbolines (ex. Harmine
Harmine is a beta-carboline and a harmala alkaloid. It occurs in a number of different plants, most notably the Syrian rue and ''Banisteriopsis caapi''. Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamin ...
, Harmaline
Harmaline is a fluorescent indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partly hydrogenated form of harmine.
Occurrence in nature
Various plants contain harmaline including ''Peganum harmala'' (Syrian rue) ...
, Tetrahydroharmine
Tetrahydroharmine (THH) is a fluorescent indole alkaloid that occurs in the tropical liana species '' Banisteriopsis caapi''.
THH, like other harmala alkaloids in ''B. caapi'', namely harmaline and harmine, is a reversible inhibitor of monoam ...
).
* Second-order modulators: (−)‐epigallocatechin‐3‐gallate.
* Non-competitive channel blockers: cicutoxin, oenanthotoxin, pentylenetetrazol
Pentylenetetrazol, also known as pentylenetetrazole, leptazol, metrazol, pentetrazol (INN), pentamethylenetetrazol, Corazol, Cardiazol, Deumacard, or PTZ, is a drug formerly used as a circulatory and respiratory stimulant. High doses cause convul ...
, picrotoxin
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Gree ...
, thujone
Thujone () is a ketone and a monoterpene that occurs predominantly in two diastereomeric (epimeric) forms: (−)-α-thujone and (+)-β-thujone.
Though it is best known as a chemical compound in the spirit absinthe, it is unlikely to be responsib ...
, and lindane
Lindane, also known as ''gamma''-hexachlorocyclohexane (γ-HCH), gammaxene, Gammallin and benzene hexachloride (BHC), is an organochlorine chemical and an isomer of hexachlorocyclohexane that has been used both as an agricultural insecticide and ...
.
Effects
Ligands which contribute to receptor activation typically haveanxiolytic
An anxiolytic (; also antipanic or antianxiety agent) is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxi ...
, anticonvulsant
Anticonvulsants (also known as antiepileptic drugs or recently as antiseizure drugs) are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of b ...
, amnesic
Amnesia is a deficit in memory caused by brain damage or disease,Gazzaniga, M., Ivry, R., & Mangun, G. (2009) Cognitive Neuroscience: The biology of the mind. New York: W.W. Norton & Company. but it can also be caused temporarily by the use ...
, sedative
A sedative or tranquilliser is a substance that induces sedation by reducing irritability or excitement. They are CNS depressants and interact with brain activity causing its deceleration. Various kinds of sedatives can be distinguished, but t ...
, hypnotic
Hypnotic (from Greek ''Hypnos'', sleep), or soporific drugs, commonly known as sleeping pills, are a class of (and umbrella term for) psychoactive drugs whose primary function is to induce sleep (or surgical anesthesiaWhen used in anesthesia ...
, euphoriant
Euphoria ( ) is the experience (or affect) of pleasure or excitement and intense feelings of well-being and happiness. Certain natural rewards and social activities, such as aerobic exercise, laughter, listening to or making music and danci ...
, and muscle relaxant
A muscle relaxant is a drug that affects skeletal muscle function and decreases the muscle tone. It may be used to alleviate symptoms such as muscle spasms, pain, and hyperreflexia. The term "muscle relaxant" is used to refer to two major therap ...
properties. Some such as muscimol
Muscimol (also known as agarin or pantherine) is one of the principal psychoactive constituents of '' Amanita muscaria'' and related species of mushroom. Muscimol is a potent and selective orthosteric agonist for the GABAA receptors and displa ...
and the z-drugs
Nonbenzodiazepines (), sometimes referred to colloquially as Z-drugs (as many of their names begin with the letter "z"), are a class of psychoactive drugs that are very benzodiazepine-like in nature. They are used in the treatment of sleep proble ...
may also be hallucinogenic
Hallucinogens are a large, diverse class of psychoactive drugs that can produce altered states of consciousness characterized by major alterations in thought, mood, and perception as well as other changes. Most hallucinogens can be categorized ...
. Ligands which decrease receptor activation usually have opposite effects, including anxiogenesis and convulsion. Some of the subtype-selective negative allosteric modulators such as α5IA are being investigated for their nootropic
Nootropics ( , or ) (colloquial: smart drugs and cognitive enhancers, similar to adaptogens) are a wide range of natural or synthetic dietary supplement, supplements or drugs and other substances that are claimed to improve cognitive function ...
effects, as well as treatments for the unwanted side effects of other GABAergic drugs.
Novel drugs
A useful property of the many benzodiazepine site allosteric modulators is that they may display selective binding to particular subsets of receptors comprising specific subunits. This allows one to determine which GABAA receptor subunit combinations are prevalent in particular brain areas and provides a clue as to which subunit combinations may be responsible for behavioral effects of drugs acting at GABAA receptors. These selective ligands may have pharmacological advantages in that they may allow dissociation of desired therapeutic effects from undesirable side effects. Few subtype selective ligands have gone into clinical use as yet, with the exception ofzolpidem
Zolpidem, sold under the brand name Ambien, among others, is a medication primarily used for the short-term treatment of sleeping problems. Guidelines recommend that it be used only after cognitive behavioral therapy for insomnia and behavior ...
which is reasonably selective for α1, but several more selective compounds are in development such as the α3-selective drug adipiplon
Adipiplon (developmental code name NG2-73) is an anxiolytic drug developed by Neurogen Corporation. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.
Adipiplon is ...
. There are many examples of subtype-selective compounds which are widely used in scientific research, including:
* CL-218,872 (highly α1-selective agonist)
* bretazenil (subtype-selective partial agonist)
* imidazenil
Imidazenil is an experimental anxiolytic drug which is derived from the benzodiazepine family, and is most closely related to other imidazobenzodiazepines such as midazolam, flumazenil, and bretazenil.
Imidazenil is a highly potent benzodiaze ...
and L-838,417
L-838,417 is an anxiolytic drug used in scientific research. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic. The compound was developed by Merck, Sharp and Dohme ...
(both partial agonists at some subtypes, but weak antagonists at others)
* QH-ii-066 (full agonist highly selective for α5 subtype)
* α5IA (selective inverse agonist for α5 subtype)
* SL-651,498
SL651498 is an anxiolytic and anticonvulsant drug used in scientific research, with a chemical structure most closely related to β-carboline derivatives such as abecarnil and gedocarnil. It has similar effects to benzodiazepine drugs, but is ...
(full agonist at α2 and α3 subtypes, and as a partial agonist at α1 and α5
* 3-acyl-4-quinolones: selective for α1 over α3
Paradoxical reactions
There are multiple indications thatparadoxical reaction A paradoxical reaction (or paradoxical effect) is an effect of a chemical substance, such as a medical drug, that is opposite to what would usually be expected. An example of a paradoxical reaction is pain caused by a pain relief medication.
Parad ...
s upon – for example – benzodiazepines, barbiturates, inhalational anesthetic
An inhalational anesthetic is a chemical compound possessing general anesthetic properties that can be delivered via inhalation. They are administered through a face mask, laryngeal mask airway or tracheal tube connected to an anesthetic vapo ...
s, propofol, neurosteroids, and alcohol are associated with structural deviations of GABAA receptors. The combination of the five subunits of the receptor (see images above) can be altered in such a way that for example the receptor's response to GABA remains unchanged but the response to one of the named substances is dramatically different from the normal one.
There are estimates that about 2–3 % of the general population may suffer from serious emotional disorders due to such receptor deviations, with up to 20% suffering from moderate disorders of this kind. It is generally assumed that the receptor alterations are, at least partly, due to genetic and also epigenetic deviations. There are indication that the latter may be triggered by, among other factors, social stress
Social stress is stress that stems from one's relationships with others and from the social environment in general. Based on the appraisal theory of emotion, stress arises when a person evaluates a situation as personally relevant and perceives t ...
or occupational burnout
According to the World Health Organization (WHO), occupational burnout is a syndrome resulting from chronic work-related stress, with symptoms characterized by "feelings of energy depletion or exhaustion; increased mental distance from one’s ...
.
See also
*4-Iodopropofol
4-Iodopropofol is a drug derived from the commonly used sedative anaesthetic agent, propofol. 4-Iodopropofol has similar effects to propofol on isolated receptors, acting primarily as a GABAA positive modulator and sodium channel blocker, but ...
* GABA receptor
The GABA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), the chief inhibitory compound in the mature vertebrate central nervous system. There are two classes of GABA receptors: GABAA and ...
* GABAB receptor
* GABAA-ρ receptor
* Gephyrin
Gephyrin is a protein that in humans is encoded by the ''GPHN'' gene.
This gene encodes a neuronal assembly protein that anchors inhibitory neurotransmitter receptors to the postsynaptic cytoskeleton via high affinity binding to a receptor subu ...
* Glycine receptor
The glycine receptor (abbreviated as GlyR or GLR) is the receptor of the amino acid neurotransmitter glycine. GlyR is an ionotropic receptor that produces its effects through chloride current. It is one of the most widely distributed inhibitory ...
* GABAA receptor positive allosteric modulators
* GABAA receptor negative allosteric modulators
References
Further reading
* * * *External links
* {{DEFAULTSORT:Gabaa Receptor Transmembrane receptors Ion channels GABA