|
Membrane Potential
Membrane potential (also transmembrane potential or membrane voltage) is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges to move from the internal to exterior cellular environments and vice versa, as long as there is no acquisition of kinetic energy or the production of radiation. The concentration gradients of the charges directly determine this energy requirement. For the exterior of the cell, typical values of membrane potential, normally given in units of milli volts and denoted as mV, range from –80 mV to –40 mV. All animal cells are surrounded by a membrane composed of a lipid bilayer with proteins embedded in it. The membrane serves as both an insulator and a diffusion barrier to the movement of ions. Transmembrane proteins, also known as ion transporter or ion pump proteins, actively push ions across the membrane and establish concentrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basis Of Membrane Potential2
Basis may refer to: Finance and accounting * Adjusted basis, the net cost of an asset after adjusting for various tax-related items *Basis point, 0.01%, often used in the context of interest rates * Basis trading, a trading strategy consisting of the purchase of a security and the sale of a similar security ** Basis of futures, the value differential between a future and the spot price ** Basis (options), the value differential between a call option and a put option ** Basis swap, an interest rate swap *Cost basis, in income tax law, the original cost of property adjusted for factors such as depreciation * Tax basis, cost of an asset and technology * Basis function *Basis (linear algebra) ** Dual basis **Orthonormal basis **Schauder basis *Basis (universal algebra) * Basis of a matroid *Generating set of an ideal: **Gröbner basis ** Hilbert's basis theorem *Generating set of a group *Base (topology) *Change of basis * Greedoid *Normal basis * Polynomial basis * Radial basis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
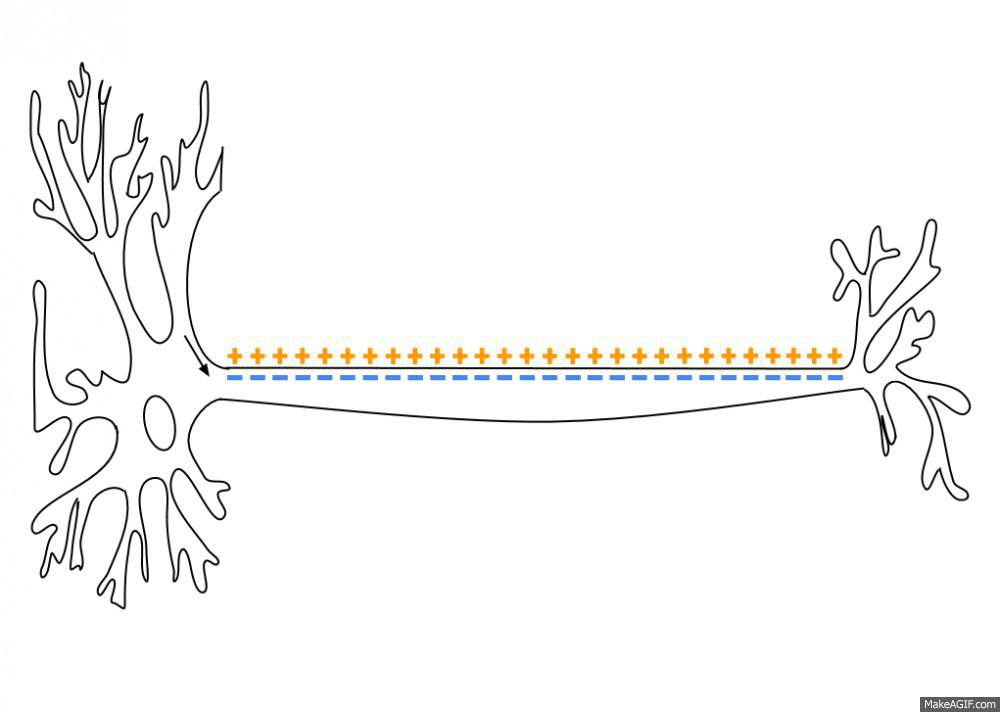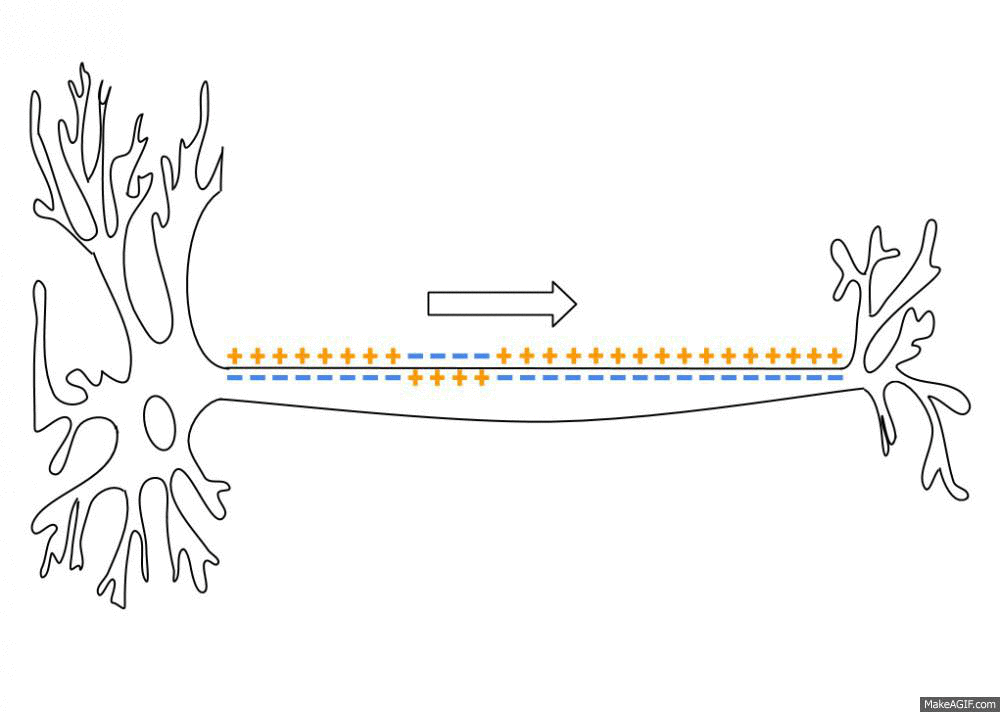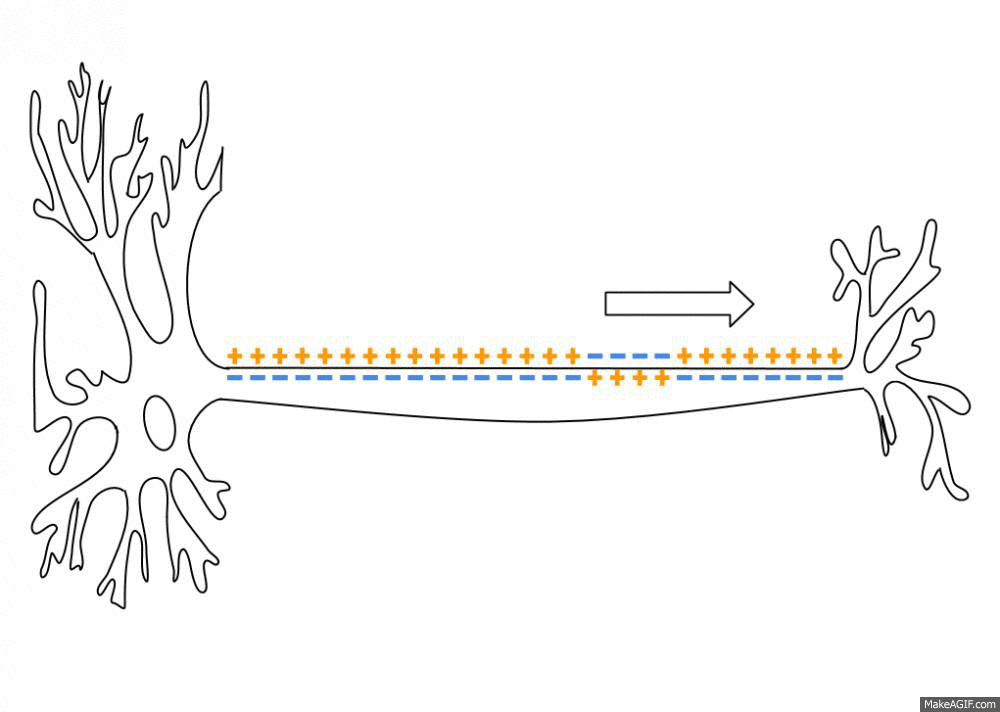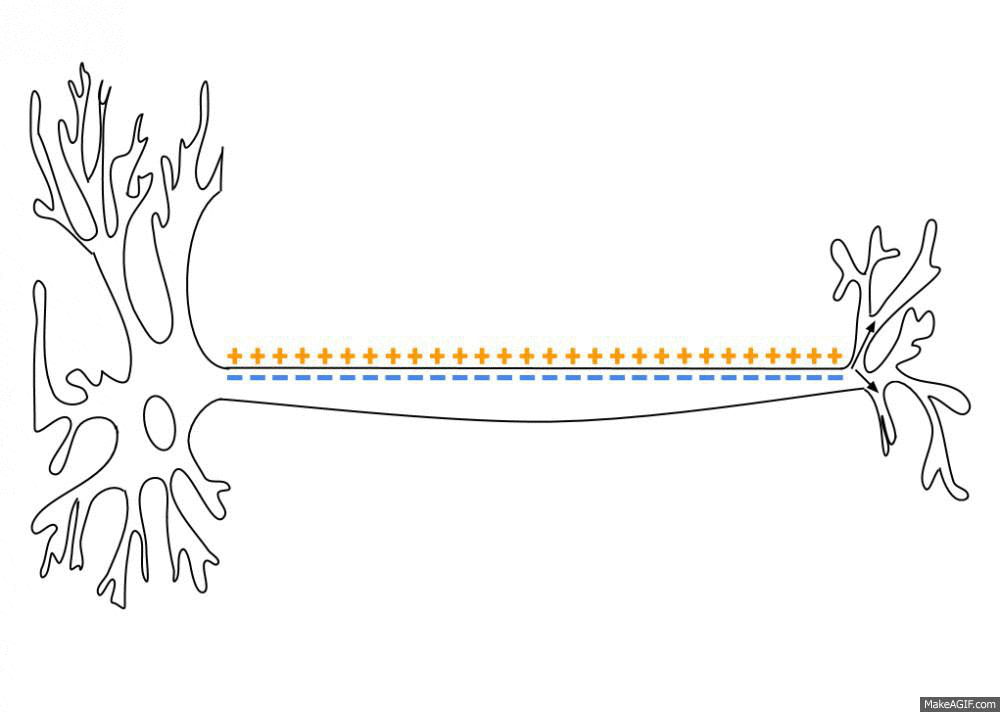
Action Potential
An action potential occurs when the membrane potential of a specific cell location rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and in some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells. In neurons, action potentials play a central role in cell-cell communication by providing for—or with regard to saltatory conduction, assisting—the propagation of signals along the neuron's axon toward synaptic boutons situated at the ends of an axon; these signals can then connect with other neurons at synapses, or to motor cells or glands. In other types of cells, their main function is to activate intracellular processes. In muscle cells, for example, an action potential is the first step in the chain of event ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much lar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theodore Holmes Bullock
Theodore Holmes Bullock (16 May 1915 – 20 December 2005) is one of the founding fathers of neuroethology. During a career spanning nearly seven decades, this American academic was esteemed both as a pioneering and influential neuroscientist, examining the physiology and evolution of the nervous system across organizational levels, and as a champion of the comparative approach, studying species from nearly all major animal groups— coelenterates, annelids, arthropods, echinoderms, molluscs, and chordates. Bullock discovered the pit organ in pit vipers and electroreceptors in weakly electric fish, as well as other electrosensory animals. His work on the jamming avoidance response in electric fish (work later carried on by Walter Heiligenberg) is an excellent example of how motor programs are integrated with incoming sensory information when generating a behavior pattern in response to a stimulus. Bullock appealed to the scientific community to look beyond established paradigms in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide ( lye) is used in soap manufacture, and sodium chloride ( edible salt) is a de-icing agent and a nutrient for animals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition. The concept of diffusion is widely used in many fields, including physics (particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection. A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature with the change in another variable, usually distance. A change in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gradient
In vector calculus, the gradient of a scalar-valued differentiable function of several variables is the vector field (or vector-valued function) \nabla f whose value at a point p is the "direction and rate of fastest increase". If the gradient of a function is non-zero at a point , the direction of the gradient is the direction in which the function increases most quickly from , and the magnitude of the gradient is the rate of increase in that direction, the greatest absolute directional derivative. Further, a point where the gradient is the zero vector is known as a stationary point. The gradient thus plays a fundamental role in optimization theory, where it is used to maximize a function by gradient ascent. In coordinate-free terms, the gradient of a function f(\bf) may be defined by: :df=\nabla f \cdot d\bf where ''df'' is the total infinitesimal change in ''f'' for an infinitesimal displacement d\bf, and is seen to be maximal when d\bf is in the direction of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conservative Field
In vector calculus, a conservative vector field is a vector field that is the gradient of some function (mathematics), function. A conservative vector field has the property that its line integral is path independent; the choice of any path between two points does not change the value of the line integral. Path independence of the line integral is equivalent to the vector field under the line integral being conservative. A conservative vector field is also Laplace equation for irrotational flow, irrotational; in three dimensions, this means that it has vanishing curl (mathematics), curl. An irrotational vector field is necessarily conservative provided that the domain is simply connected. Conservative vector fields appear naturally in mechanics: They are vector fields representing forces of physical systems in which energy is conservation of energy, conserved. For a conservative system, the work (physics), work done in moving along a path in a configuration space depends on only th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field for a system of charged particles. Electric fields originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, one of the four fundamental interactions (also called forces) of nature. Electric fields are important in many areas of physics, and are exploited in electrical technology. In atomic physics and chemistry, for instance, the electric field is the attractive force holding the atomic nucleus and electrons together in atoms. It is also the force responsible for chemical bonding between atoms that result in molecules. The electric field is defined as a vector field that associates to each point in space the electrostatic (Coulomb) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground (electricity)
In electrical engineering, ground or earth is a reference point in an electrical circuit from which voltages are measured, a common return path for electric current, or a direct physical connection to the Earth. Electrical circuits may be connected to ground for several reasons. Exposed conductive parts of electrical equipment are connected to ground, to protect users from electrical shock hazard. If internal insulation fails, dangerous voltages may appear on the exposed conductive parts. Connecting exposed parts to ground will allow circuit breakers (or RCDs) to interrupt power supply in the event of a fault. In electric power distribution systems, a protective earth (PE) conductor is an essential part of the safety provided by the earthing system. Connection to ground also limits the build-up of static electricity when handling flammable products or electrostatic-sensitive devices. In some telegraph and power transmission circuits, the ground itself can be used as one co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |