|
Chromophore
A chromophore is the part of a molecule responsible for its color. The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the moiety that causes a conformational change in the molecule when hit by light. Conjugated pi-bond system chromophores Just like how two adjacent p-orbitals in a molecule will form a pi-bond, three or more adjacent p-orbitals in a molecule can form a conjugated pi-system. In a conjugated pi-system, electrons are able to capture certain photons as the electrons resonate along a certain distance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision). Some microorganisms use retinal to convert light into metabolic energy. In fact, a recent study suggests most living organisms on our planet ~3 billion years ago used retinal to convert sunlight into energy rather than chlorophyll. Since retinal absorbs mostly green light and transmits purple light, this gave rise to the Purple Earth Hypothesis. There are many forms of vitamin A — all of which are converted to retinal, which cannot be made without them. Retinal itself is considered to be a form of vitamin A when eaten by an animal. The number of different molecules that can be converted to retinal varies from species to species. Retinal was originally called retinene, and was renamed after it was discovered to be vitamin A aldehyde. Vertebrate animals ingest reti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele. Conjugation is the overlap of one p-orbital with another across an adjacent σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons across all the adjacent aligned p-orbitals. The π electrons do not belong to a single bond or atom, but rather to a group of atoms. Molecules containing conjugated syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele. Conjugation is the overlap of one p-orbital with another across an adjacent σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons across all the adjacent aligned p-orbitals. The π electrons do not belong to a single bond or atom, but rather to a group of atoms. Molecules containing conjugated syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PH Indicator
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH. Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor. The pH value of a neutral solution is 7.0 at 25°C ( standard laboratory conditions). Solutions with a pH value below 7.0 are considered acidic and solutions with pH value above 7.0 are basic. Since most naturally occurring organic compounds are weak electrolytes, such as carboxylic acids and amines, pH indicators find many applications in biology and analytical chemistry. Moreover, pH indicators form one of the three main ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Color
Color (American English) or colour (British English) is the visual perceptual property deriving from the spectrum of light interacting with the photoreceptor cells of the eyes. Color categories and physical specifications of color are associated with objects or materials based on their physical properties such as light absorption, reflection, or emission spectra. By defining a color space, colors can be identified numerically by their coordinates. Because perception of color stems from the varying spectral sensitivity of different types of cone cells in the retina to different parts of the spectrum, colors may be defined and quantified by the degree to which they stimulate these cells. These physical or physiological quantifications of color, however, do not fully explain the psychophysical perception of color appearance. Color science includes the perception of color by the eye and brain, the origin of color in materials, color theory in art, and the physics of electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fall Leaves (199582361)
Autumn, also known as fall in American English and Canadian English, is one of the four temperate seasons on Earth. Outside the tropics, autumn marks the transition from summer to winter, in September (Northern Hemisphere) or March (Southern Hemisphere). Autumn is the season when the duration of daylight becomes noticeably shorter and the temperature cools considerably. Day length decreases and night length increases as the season progresses until the Winter Solstice in December (Northern Hemisphere) and June (Southern Hemisphere). One of its main features in temperate climates is the striking Autumn leaf color, change in colour for the leaves of deciduous trees as they leaf#Seasonal leaf loss, prepare to shed. Date definitions Some cultures regard the autumnal equinox as "mid-autumn", while others with a longer Seasonal lag, temperature lag treat the equinox as the start of autumn. In the English-speaking world of high latitude countries, autumn traditionally began with Lam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthocyanins
Anthocyanins (), also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart gave the name Anthokyan to a chemical compound that gives flowers a blue color for the first time in his treatise "''Die Farben der Blüthen''". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins. Anthocyanins belong to a parent class of molecules called flavonoids synthesized via the phenylpropanoid pathway. They occur in all tissues of higher plants, including leaves, stems, roots, flowers, and fruits. Anthocyanins are derived from anthocyanidins by adding sugars. They are odorless and moderately astringent. Although approved as food and beverage colorant in the European Union, anthocyanins are not approved for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carotene
The term carotene (also carotin, from the Latin ''carota'', "carrot") is used for many related unsaturated hydrocarbon substances having the formula C40Hx, which are synthesized by plants but in general cannot be made by animals (with the exception of some aphids and spider mites which acquired the synthesizing genes from fungi). Carotenes are photosynthetic pigments important for photosynthesis. Carotenes contain no oxygen atoms. They absorb ultraviolet, violet, and blue light and scatter orange or red light, and (in low concentrations) yellow light. Carotenes are responsible for the orange colour of the carrot, after which this class of chemicals is named, and for the colours of many other fruits, vegetables and fungi (for example, sweet potatoes, chanterelle and orange cantaloupe melon). Carotenes are also responsible for the orange (but not all of the yellow) colours in dry foliage. They also (in lower concentrations) impart the yellow coloration to milk-fat and butter. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lycopene
Lycopene is an organic compound classified as a tetraterpene and a carotene. Lycopene (from the neo-Latin ''Lycopersicum'', the tomato species) is a bright red carotenoid hydrocarbon found in tomatoes and other red fruits and vegetables. Occurrence Aside from tomatoes, it is found in red carrots, watermelons, grapefruits, and papayas. It is not present in strawberries or cherries. It has no vitamin A activity. In plants, algae, and other photosynthetic organisms, lycopene is an intermediate in the biosynthesis of many carotenoids, including beta-carotene, which is responsible for yellow, orange, or red pigmentation, photosynthesis, and photoprotection. Like all carotenoids, lycopene is a tetraterpene. It is insoluble in water. Eleven conjugated double bonds give lycopene its deep red color. Owing to the strong color, lycopene is useful as a food coloring (registered as E160d) and is approved for use in the US, Australia and New Zealand (registered as 160d) and the European ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
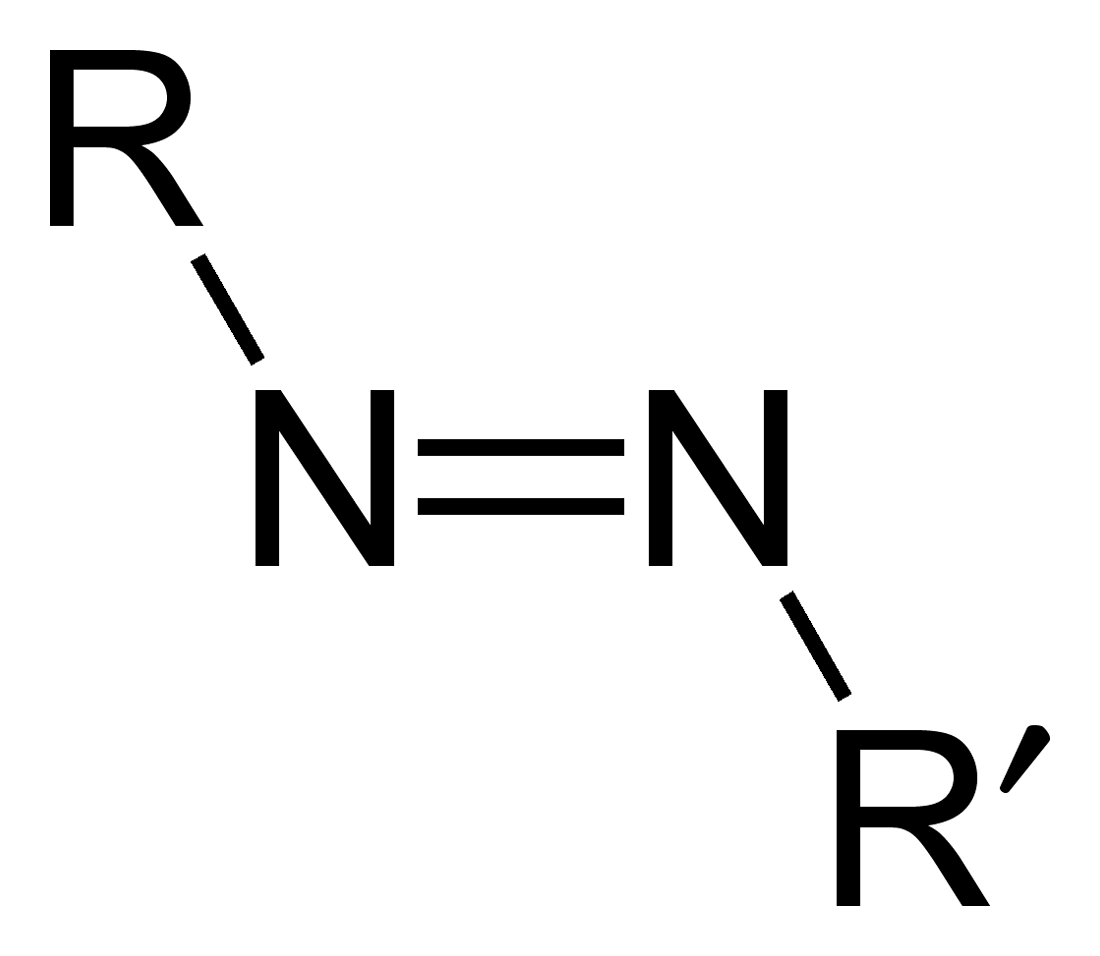
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Coloring
Food coloring, or color additive, is any dye, pigment, or substance that imparts color when it is added to food or drink. They come in many forms consisting of liquids, powders, gels, and pastes. Food coloring is used in both commercial food production and domestic cooking. Food colorants are also used in a variety of non-food applications, including cosmetics, pharmaceuticals, home craft projects, and medical devices. Purpose of food coloring People associate certain colors with certain flavors, and the color of food can influence the perceived flavor in anything from candy to wine. Sometimes, the aim is to simulate a color that is perceived by the consumer as natural, such as adding red coloring to glacé cherries (which would otherwise be beige), but sometimes it is for effect, like the green ketchup that Heinz launched in 2000. Color additives are used in foods for many reasons including: * To make food more attractive, appealing, appetizing, and informative * Offset c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpeg/1200px-Fall_Leaves_(199582361).jpeg)