|
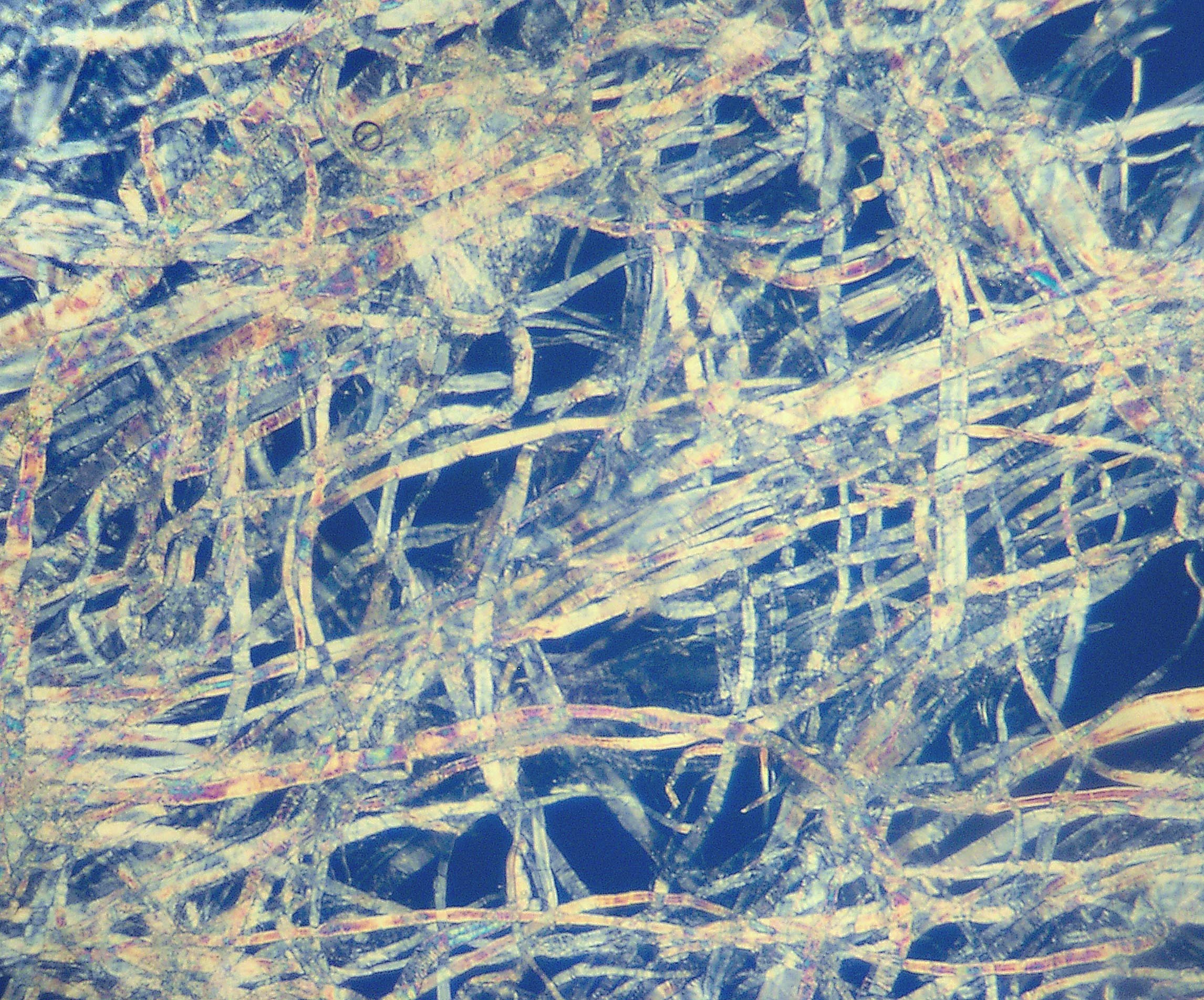
Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%. Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton. Some animals, particularly ruminants and termites, can digest cellulose with the help of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rayon
Rayon is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. It is also called viscose. Many types and grades of viscose fibers and films exist. Some imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk. The fibre is used to make textiles for clothing and other purposes. Rayon production involves solubilizing cellulose to allow turning the fibers into required form. Three common ways to solubilize are the cuprammonium process, not in use today, using ammoniacal solutions of copper salts; the viscose process, the most common today, using alkali and carbon sulfide; and the Lyocell process, using amine oxide. The last avoids the neurotoxic carbon sulfide of the viscose process but is also more expensive. Rayon and its variants Rayon is produced by dissolving cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides, or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellophane
Cellophane is a thin, transparent sheet made of regenerated cellulose. Its low permeability to air, oils, greases, bacteria, and liquid water makes it useful for food packaging. Cellophane is highly permeable to water vapour, but may be coated with nitrocellulose lacquer to prevent this. Cellophane is also used in transparent pressure-sensitive tape, tubing and many other similar applications. Cellophane is compostable and biodegradable, and can be obtained from biomaterials. Production, however, uses carbon disulfide (CS2), which has been found to be highly toxic to workers. The lyocell process, however, can be used to produce cellulose film without involving carbon disulfide. "Cellophane" is a generic term in some countries, while in other countries it is a registered trademark. Production Cellulose from wood, cotton, hemp, or other sources is dissolved in alkali and carbon disulfide to make a solution called viscose, which is then extruded through a slit into a bath of dilu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellulosic Ethanol
Cellulosic ethanol is ethanol (ethyl alcohol) produced from cellulose (the stringy fiber of a plant) rather than from the plant's seeds or fruit. It can be produced from grasses, wood, algae, or other plants. It is generally discussed for use as a biofuel. The carbon dioxide that plants absorb as they grow offsets some of the carbon dioxide emitted when ethanol made from them is burned, so cellulosic ethanol fuel has the potential to have a lower carbon footprint than fossil fuels. Interest in cellulosic ethanol is driven by its potential to replace ethanol made from corn or sugarcane. Since these plants are also used for food products, diverting them for ethanol production can cause food prices to rise; cellulose-based sources, on the other hand, generally do not compete with food, since the fibrous parts of plants are mostly inedible to humans. Another potential advantage is the high diversity and abundance of cellulose sources; grasses, trees and algae are found in almost ever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cotton
Cotton is a soft, fluffy staple fiber that grows in a boll, or protective case, around the seeds of the cotton plants of the genus ''Gossypium'' in the mallow family Malvaceae. The fiber is almost pure cellulose, and can contain minor percentages of waxes, fats, pectins, and water. Under natural conditions, the cotton bolls will increase the dispersal of the seeds. The plant is a shrub native to tropical and subtropical regions around the world, including the Americas, Africa, Egypt and India. The greatest diversity of wild cotton species is found in Mexico, followed by Australia and Africa. Cotton was independently domesticated in the Old and New Worlds. The fiber is most often spun into yarn or thread and used to make a soft, breathable, and durable textile. The use of cotton for fabric is known to date to prehistoric times; fragments of cotton fabric dated to the fifth millennium BC have been found in the Indus Valley civilization, as well as fabric remnants dated back ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose and alginate. Other examples of biopolymers include natural rubbers (polymers of isoprene), suberin and lignin (complex polyphenolic polymers), cutin and cutan (complex polymers of long-chain fatty acids) and melanin. In addition to their many essential roles in living organisms, biopolymers have applications in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Wall
A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mechanism. Cell walls are absent in many eukaryotes, including animals, but are present in some other ones like fungi, algae and plants, and in most prokaryotes (except mollicute bacteria). A major function is to act as pressure vessels, preventing over-expansion of the cell when water enters. The composition of cell walls varies between taxonomic group and species and may depend on cell type and developmental stage. The primary cell wall of land plants is composed of the polysaccharides cellulose, hemicelluloses and pectin. Often, other polymers such as lignin, suberin or cutin are anchored to or embedded in plant cell walls. Algae possess cell walls made of glycoproteins and polysaccharides such as carrageenan and agar that are absent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Termite
Termites are small insects that live in colonies and have distinct castes (eusocial) and feed on wood or other dead plant matter. Termites comprise the infraorder Isoptera, or alternatively the epifamily Termitoidae, within the order Blattodea (along with cockroaches). Termites were once classified in a separate order from cockroaches, but recent phylogenetic studies indicate that they evolved from cockroaches, as they are deeply nested within the group, and the sister group to wood eating cockroaches of the genus ''Cryptocercus''. Previous estimates suggested the divergence took place during the Jurassic or Triassic. More recent estimates suggest that they have an origin during the Late Jurassic, with the first fossil records in the Early Cretaceous. About 3,106 species are currently described, with a few hundred more left to be described. Although these insects are often called "white ants", they are not ants, and are not closely related to ants. Like ants and some bees a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wood Pulp
Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemical or plant-based additives, pulp is the major raw material used in papermaking and the industrial production of other paper products. History Before the widely acknowledged invention of papermaking by Cai Lun in China around 105 AD, paper-like writing materials such as papyrus and amate were produced by ancient civilizations using plant materials which were largely unprocessed. Strips of bark or bast material were woven together, beaten into rough sheets, dried, and polished by hand. Pulp used in modern and traditional papermaking is distinguished by the process which produces a finer, more regular slurry of cellulose fibers which are pulled out of solution by a screen and dried to form sheets or rolls. The earliest paper produced in China consisted of bast fibers from the paper mulberr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biofuel
Biofuel is a fuel that is produced over a short time span from biomass, rather than by the very slow natural processes involved in the formation of fossil fuels, such as oil. According to the United States Energy Information Administration (EIA), biofuels are mostly used for transportation, but can also be used for heating and electricity. Biofuel can be produced from plants or from agricultural, domestic or industrial biowaste. The greenhouse gas mitigation potential of biofuel varies considerably, from emission levels comparable to fossil fuels in some scenarios to negative emissions in others. See the biomass article for more on this particular subject. The two most common types of biofuel are bioethanol and biodiesel. The U.S. is the largest producer of bioethanol, while the EU is the largest producer of biodiesel. The energy content in the global production of bioethanol and biodiesel is 2.2 and 1.8 EJ per year, respectively. * Bioethanol is an alcohol made by fermen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_in_2002.png)