|
Cannabicyclol
Cannabicyclol (CBL) is a non- psychoactive cannabinoid found in ''Cannabis''. CBL is a degradative product like cannabinol, with cannabichromene degrading into CBL through natural irradiation or under acid conditions. CBL is not scheduled under the Convention on Psychotropic Substances. See also * Cannabis * Medical cannabis References External links CTD's Cannabicyclol pagefrom the Comparative Toxicogenomics Database The Comparative Toxicogenomics Database (CTD) is a public website and research tool launched in November 2004 that curates scientific data describing relationships between chemicals/drugs, genes/proteins, diseases, taxa, phenotypes, GO annotations ... Wiley-VCH list of chemicals Phytocannabinoids Phenols Benzopyrans Cyclobutanes Cyclopentanes Heterocyclic compounds with 4 rings {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Cannabis
Medical cannabis, or medical marijuana (MMJ), is cannabis and cannabinoids that are prescribed by physicians for their patients. The use of cannabis as medicine has not been rigorously tested due to production and governmental restrictions, resulting in limited clinical research to define the safety and efficacy of using cannabis to treat diseases. Preliminary evidence has indicated that cannabis might reduce nausea and vomiting during chemotherapy and reduce chronic pain and muscle spasms. Regarding non-inhaled cannabis or cannabinoids, a 2021 review found that it provided little relief against chronic pain and sleep disturbance, and caused several transient adverse effects, such as cognitive impairment, nausea, and drowsiness. Short-term use increases the risk of minor and major adverse effects. Common side effects include dizziness, feeling tired, vomiting, and hallucinations. Long-term effects of cannabis are not clear. Concerns include memory and cognition problems, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychoactive Substance
A psychoactive drug, psychopharmaceutical, psychoactive agent or psychotropic drug is a chemical substance, that changes functions of the nervous system, and results in alterations in perception, mood, consciousness, cognition or behavior. These substances may be used medically, recreationally or spiritually to a. Purposefully improve one’s perceived performance b. Alter one's consciousness (such as with entheogens for ritual, spiritual or shamanic purposes) or c. For research. Some categories of psychoactive drugs - which are believed, by some, to have therapeutic value - may be prescribed by some physicians and other healthcare practitioners. Examples of medication categories that may contain potentially beneficial psychoactive drugs include, but are not limited to: # Anesthetics # Analgesics # Anticonvulsants # Anti-Parkinson’s medications # Medications used to treat Neuropsychiatric Disorders a. Antidepressants b. Anxiolytics c. Antipsychotics d. Sti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is a major constituent of temperate Cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four (i.e., THCA, CBDA, CBCA and their common precursor CBGA) have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea. Phytocannabinoids are multi-ring phenolic compounds structurally related to THC, but endocannabinoids are fatty acid derivatives. Nonclassical synthetic cannabinoids (cannabimimetics) include amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabis
''Cannabis'' () is a genus of flowering plants in the family Cannabaceae. The number of species within the genus is disputed. Three species may be recognized: ''Cannabis sativa'', '' C. indica'', and '' C. ruderalis''. Alternatively, ''C. ruderalis'' may be included within ''C. sativa'', all three may be treated as subspecies of ''C. sativa'', or ''C. sativa'' may be accepted as a single undivided species. The genus is widely accepted as being indigenous to and originating from Asia. The plant is also known as hemp, although this term is often used to refer only to varieties of ''Cannabis'' cultivated for non-drug use. Cannabis has long been used for hemp fibre, hemp seeds and their oils, hemp leaves for use as vegetables and as juice, medicinal purposes, and as a recreational drug. Industrial hemp products are made from cannabis plants selected to produce an abundance of fibre. Various cannabis strains have been bred, often selectively to pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinol
Cannabinol (CBN) is a mildly psychoactive cannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS). CBN was the first cannabis compound to be isolated from cannabis extract in the late 1800s. Its structure and chemical synthesis were achieved by 1940, followed by some of the first basic research studies to determine the effects of individual cannabis-derived compounds in vivo. Although CBN shares the same mechanism of action as other phytocannabinoids (e.g., delta-9 tetrahydrocannabinol or D9THC), it has a lower affinity for CB1 receptors, meaning that much higher doses of CBN are required in order to experience effects, such as mild sedation. Chemical structure Cannabinoid receptor agonists are categorized into four groups based on chemical structure. CBN, as one of the many phytocannabinoids derived from Cannabis Sativa L, is considered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
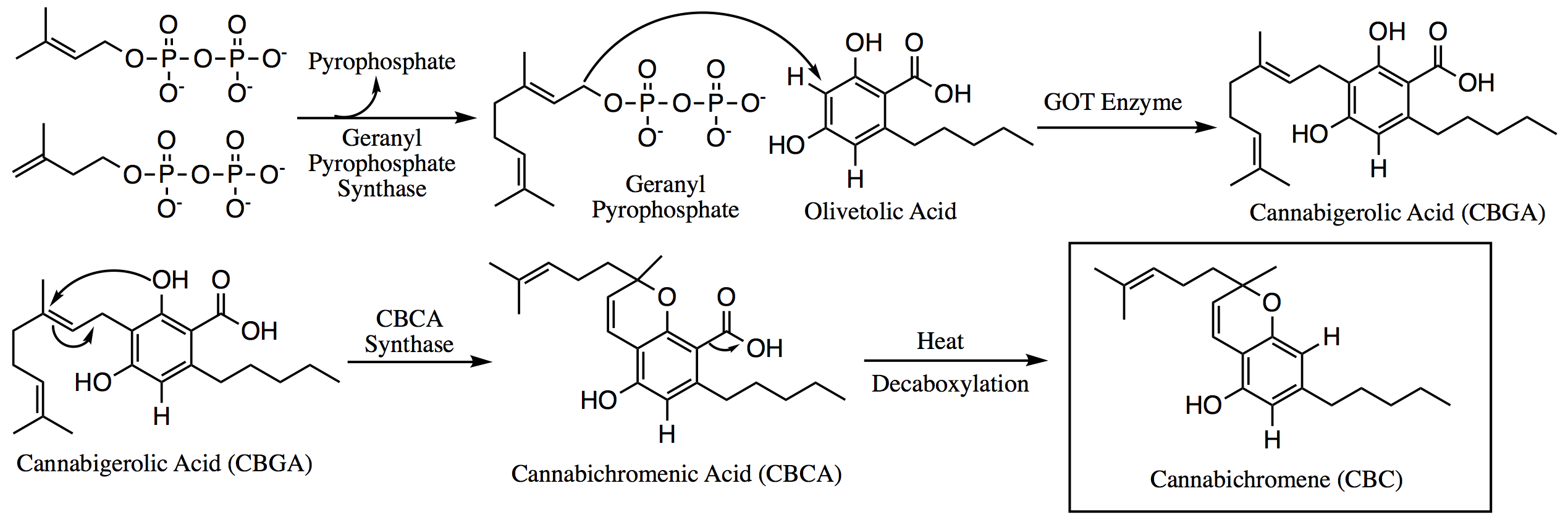
Cannabichromene
Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, is an anti-inflammatory which may contribute to the pain-killing effect of cannabis. It is one of the hundreds of cannabinoids found in the ''Cannabis'' plant, and is therefore a phytocannabinoid. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and its derivatives are as abundant as cannabinols in cannabis. It is not scheduled by the Convention on Psychotropic Substances. It is more common in tropical cannabis varieties. Biosynthesis Within the ''Cannabis'' plant, CBC occurs mainly as cannabichromenic acid (CBCA, 2-COOH-CBC, CBC-COOH). Geranyl pyrophosphate and olivetolic acid combine to produce cannabigerolic acid (CBGA; the sole intermediate for all other phytocannabinoids), which is cyclized by the enzyme CBCA synthas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convention On Psychotropic Substances
The Convention on Psychotropic Substances of 1971 is a United Nations treaty designed to control psychoactive drugs such as #Amphetamine-type stimulants, amphetamine-type stimulants, barbiturates, benzodiazepines, and Psychedelic drug, psychedelics signed in Vienna, Austria on 21 February 1971. The Single Convention on Narcotic Drugs of 1961 did not ban the many newly discovered psychotropics, since its scope was limited to drugs with Cannabis (drug), cannabis, coca and opium-like effects. During the 1960s such recreational drug use, drugs became widely available, and government authorities opposed this for numerous reasons, arguing that along with negative health effects, drug use led to lowered moral standards. The Convention, which contains import and export restrictions and other rules aimed at limiting drug use to scientific and medical purposes, came into force on 16 August 1976. As of 2013, 183 member states of the United Nations, member states are Parties to the treaty. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabis (drug)
Cannabis, also known as marijuana among List of names for cannabis, other names, is a psychoactive drug from the cannabis plant. Native to Central or South Asia, the cannabis plant has been used as a drug for both Recreational marijuana, recreational and Entheogenic use of cannabis, entheogenic purposes and in various traditional medicines for centuries. Tetrahydrocannabinol (THC) is the main psychoactive component of cannabis, which is one of the 483 known compounds in the plant, including at least 65 other cannabinoids, such as cannabidiol (CBD). Cannabis can be used by Cannabis smoking, smoking, Vaporizer (inhalation device), vaporizing, Cannabis edible, within food, or Tincture of cannabis, as an extract. Cannabis has various effects of cannabis, mental and physical effects, which include euphoria, altered states of mind and Cannabis and time perception, sense of time, difficulty concentrating, Cannabis and memory, impaired short-term memory, impaired motor skill, body mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comparative Toxicogenomics Database
The Comparative Toxicogenomics Database (CTD) is a public website and research tool launched in November 2004 that curates scientific data describing relationships between chemicals/drugs, genes/proteins, diseases, taxa, phenotypes, GO annotations, pathways, and interaction modules. The database is maintained by the Department of Biological Sciences at North Carolina State University. Background The Comparative Toxicogenomics Database (CTD) is a public website and research tool that curates scientific data describing relationships between chemicals, genes/proteins, diseases, taxa, phenotypes, GO annotations, pathways, and interaction modules, launched on November 12, 2004. The database is maintained by the Department of Biological Sciences at North Carolina State University. Goals and objectives One of the primary goals of CTD is to advance the understanding of the effects of environmental chemicals on human health on the genetic level, a field called toxicogenomics. The etiol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytocannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is a major constituent of temperate Cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four (i.e., THCA, CBDA, CBCA and their common precursor CBGA) have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea. Phytocannabinoids are multi-ring phenolic compounds structurally related to THC, but endocannabinoids are fatty acid derivatives. Nonclassical synthetic cannabinoids (cannabimimetics) include amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzopyrans
Benzopyran is a polycyclic organic compound that results from the fusion of a benzene ring to a heterocyclic pyran ring. According to current IUPAC nomenclature, the name chromene used in previous recommendations is retained; however, systematic ‘benzo’ names, for example 2''H''-1-benzopyran, are preferred IUPAC names for chromene, isochromene, chromane, isochromane, and their chalcogen analogues. There are two isomers of benzopyran that vary by the orientation of the fusion of the two rings compared to the oxygen, resulting in 1-benzopyran (chromene) and 2-benzopyran (isochromene)—the number denotes where the oxygen atom is located by standard naphthalene-like nomenclature. Some benzopyrans have shown anticancerous activity ''in vitro''. The radical form of benzopyran is paramagnetic. The unpaired electron is delocalized over the whole benzopyran molecule, rendering it less reactive than one would expect otherwise. A similar example is the cyclopentadienyl radical. Commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)