Sulfolane on:
[Wikipedia]
[Google]
[Amazon]
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a
 Shortly thereafter, it was discovered that both the product yield and the lifetime of the
Shortly thereafter, it was discovered that both the product yield and the lifetime of the
vapor pressureliquid densitydynamic liquid viscositysurface tension
of sulfolane
Typical Properties of High Purity SulfolaneSulfolane: A Versatile Dipolar Aprotic Solvent (1 of 4), sponsored by NovasolA Continuous Protodecarboxylation of Heteroaromatic Carboxylic Acids in Sulfolane (2 of 4), article sponsored by Novasol
Solvents Sulfones Sulfur heterocycles Heterocyclic compounds with 1 ring
solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
for extractive distillation and chemical reactions. Sulfolane was originally developed by the Shell Oil Company
Shell USA, Inc. (formerly Shell Oil Company, Inc.) is the United States-based wholly owned subsidiary of Shell plc, a UK-based transnational corporation " oil major" which is amongst the largest oil companies in the world. Approximately 18,0 ...
in the 1960s as a solvent to purify butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two v ...
. Sulfolane is a polar aprotic solvent, and it is readily soluble in water.
Properties
Sulfolane is classified as a sulfone, a group of organosulfur compounds containing a sulfonylfunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
. The sulfone group is a sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
atom doubly bonded to two oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
atoms and singly bonded to two carbon centers. The sulfur-oxygen double bond is polar, conferring good solubility in water, while the four carbon ring provides non-polar stability. These properties allow it to be miscible in both water and hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s, resulting in its widespread use as a solvent for purifying hydrocarbon mixtures.
Synthesis
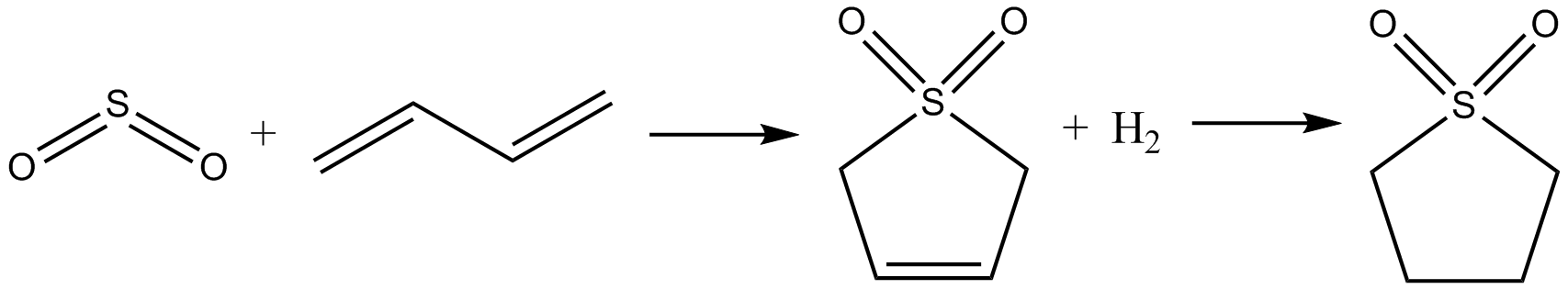
The original method developed by theShell Oil Company
Shell USA, Inc. (formerly Shell Oil Company, Inc.) is the United States-based wholly owned subsidiary of Shell plc, a UK-based transnational corporation " oil major" which is amongst the largest oil companies in the world. Approximately 18,0 ...
was to first allow butadiene to react with sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic a ...
via a cheletropic reaction to give sulfolene
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source ...
. This was then hydrogenated using Raney nickel as a catalyst to give sulfolane.Hillis O. Folkins, "Benzene" in ''Ullmann’s Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2005.
 Shortly thereafter, it was discovered that both the product yield and the lifetime of the
Shortly thereafter, it was discovered that both the product yield and the lifetime of the catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
could be improved by adding hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
and then neutralizing to a pH of roughly 5-8 before hydrogenation. Developments have continued over the years, including in the catalysts used. Recently, it was found that Ni-B/MgO showed superior catalytic activity to that of Raney nickel and other common catalysts that have been used in the hydrogenation of sulfolene.
Other syntheses have also been developed, such as oxidizing tetrahydrothiophene
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorle ...
with hydrogen peroxide. This reaction produces tetramethylene sulfoxide, which can then be further oxidized. Because the first oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
occurs at low temperature and the second at higher temperature, the reaction can be controlled at each stage. This gives greater freedom for the manipulation of the reaction, which can potentially lead to higher yields and purity.
Uses
Sulfolane is widely used as an industrialsolvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
, especially in the extraction of aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
hydrocarbons from hydrocarbon mixtures and to purify natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
. The first large scale commercial use of sulfolane, the sulfinol process, was first implemented by Shell Oil Company
Shell USA, Inc. (formerly Shell Oil Company, Inc.) is the United States-based wholly owned subsidiary of Shell plc, a UK-based transnational corporation " oil major" which is amongst the largest oil companies in the world. Approximately 18,0 ...
in March 1964 at the Person gas plant near Karnes City, Texas. The sulfinol process purifies natural gas by removing H2S, CO2, COS and mercaptans from natural gas with a mixture of alkanolamine and sulfolane.
Shortly after the sulfinol process was implemented, sulfolane was found to be highly effective in separating high purity aromatic compounds from hydrocarbon mixtures using liquid-liquid extraction. This process is widely used in refineries and the petrochemical industry
The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics (polymer) industry. It directly interfaces with the petroleum industry, especially the downstream sector.
Comp ...
. Because sulfolane is one of the most efficient industrial solvents for purifying aromatics, the process operates at a relatively low solvent-to-feed ratio, making sulfolane relatively cost effective compared to similar-purpose solvents. In addition, it is selective in a range that complements distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
; where sulfolane cannot separate two compounds, distillation easily can and vice versa, keeping sulfolane units useful for a wide range of compounds with minimal additional cost.
Whereas sulfolane is highly stable and can therefore be reused many times, it does eventually degrade into acidic byproducts. A number of measures have been developed to remove these byproducts, allowing the sulfolane to be reused and increase the lifetime of a given supply. Some methods that have been developed to regenerate spent sulfolane include vacuum and steam distillation, back extraction, adsorption, and anion-cation exchange resin columns.
Sulfolane is also added to hydrofluoric acid as a vapor suppressant, commonly for use in a refinery's alkylation unit. This "modified" hydrofluoric acid is less prone to vaporization if released in its liquid form.
As a pollutant
Groundwater in parts of the city of North Pole, Alaska, has been contaminated with sulfolane due to pollution from a now-closed petroleum refinery. Due to this contamination, affected residents have been supplied with alternative potable water sources. Animal studies on the toxicity of sulfolane are ongoing, funded through the US federal government's National Toxicology Program. No long-term ''in vivio'' animal studies have been done, which prevents any firm conclusion as to whether sulfolane is a carcinogen, although ''in vitro'' studies have failed to demonstrate any cancerous changes in bacterial or animal cells. In animal studies, high doses of sulfolane have induced negative impacts on the central nervous system, including hyperactivity, convulsions and hypothermia; the impacts of lower doses, especially over the long-term, are still being studied.See also
*Sulfolene
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The compound is used as a source ...
*Tetrahydrothiophene
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorle ...
* Methylsulfonylmethane
References
{{reflist *Ge, Shaohui; Wu, Zhijie; Zhang, Minghui; Li, Wei; Tao, Keyi.''Industrial & Engineering Chemistry Research'',2006''45(7)'', 2229-2234, *Sharipov, A. Kh.''Russian Journal of Applied Chemistry''2003,''76(1)'', 108-113. *Dunn, C. L.; Freitas, E. R.; Hill, E. S.; Sheeler, J. E. R., Jr. Proc., Ann. Conv. Nat. Gas Processors Assoc. Am.,''Tech. Papers''1965,''44'' 55-8 *Broughton, Donald B.; Asselin, George F. UOP Process Div., Universal Oil Prod. Co., Des Plaines, IL, USA. World Petrol. Congr., Proc., 7th1968, Meeting Date 1967,''4'' 65-73. Publisher: Elsevier Publ. Co. Ltd., Barking, Engl *Lal, Raj Kumar Jagadamba; Bhat, Sodankoor Garadi Thirumaleshwara. (Indian Petrochemicals Corp. Ltd., India). Eur. Pat. Appl. 1989-308019 (1991) *Van der Wiel, A.''Nature''1960,''187'' 142-3. *Block, E.''Reactions of Organosulfur Compounds''; Academic: New York, 1978 *Belen'kii, L.I.''Chemistry of Organosulfur Compounds''; Horwood: New York, 1990External links
* Calculation ovapor pressure
of sulfolane
Typical Properties of High Purity Sulfolane
Solvents Sulfones Sulfur heterocycles Heterocyclic compounds with 1 ring