|
Tetrahydrothiophene
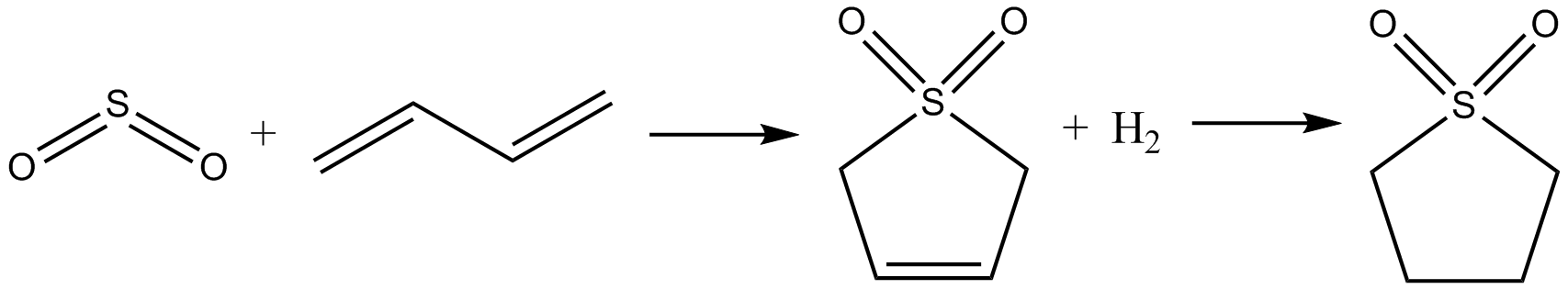
Tetrahydrothiophene is an organosulfur compound with the formula (CH2)4S. The molecule consists of a five-membered saturated ring with four methylene groups and a sulfur atom. It is the saturated analog of thiophene. It is a volatile, colorless liquid with an intensely unpleasant odor. It is also known as thiophane, thiolane, or THT. While THT is not particularly common, the vitamin biotin is essential for life in aerobic organisms. Synthesis and reactions Tetrahydrothiophene is prepared by the reaction of tetrahydrofuran with hydrogen sulfide. This vapor-phase reaction is catalyzed by alumina and other heterogenous acid catalysts. This compound is a ligand in coordination chemistry, an example being the complex chloro(tetrahydrothiophene)gold(I). Oxidation of THT gives the sulfone sulfolane, which is of interest as a polar, odorless solvent: : Sulfolane is, however, more conventionally prepared from butadiene. Natural occurrence Both unsubstituted and substituted tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfolane
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solvent for extractive distillation and chemical reactions. Sulfolane was originally developed by the Shell Oil Company in the 1960s as a solvent to purify butadiene. Sulfolane is a polar aprotic solvent, and it is readily soluble in water. Properties Sulfolane is classified as a sulfone, a group of organosulfur compounds containing a sulfonyl functional group. The sulfone group is a sulfur atom doubly bonded to two oxygen atoms and singly bonded to two carbon centers. The sulfur-oxygen double bond is polar, conferring good solubility in water, while the four carbon ring provides non-polar stability. These properties allow it to be miscible in both water and hydrocarbons, resulting in its widespread use as a solvent for purifying hydrocarbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloro(tetrahydrothiophene)gold(I)
Chloro(tetrahydrothiophene)gold(I), abbreviated (tht)AuCl, is a coordination complex of gold Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me .... Like the dimethyl sulfide analog, this compound is used as a entry point to gold chemistry. The tetrahydrothiophene ligand is labile and is readily substituted with other stronger ligands. Preparation This compound may be prepared by the reduction of tetrachloroauric acid with tetrahydrothiophene: : The complex adopts a linear coordination geometry, as is typical of many gold(I) compounds. It crystallizes in the orthorhombic space group ''Pmc''21 with a = 6.540(1) Å, b = 8.192(1) Å, c = 12.794(3) Å with Z = 4 formula units per unit cell. The bromide congener is isostructural. It is somewhat less thermally labile compared to (Me2S)A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eruca Sativa
Arugula (American English) or rocket (Commonwealth English) (''Eruca vesicaria''; syns. ''Eruca sativa'' Mill., ''E. vesicaria'' subsp. ''sativa'' (Miller) Thell., ''Brassica eruca'' L.) is an edible annual plant in the family Brassicaceae used as a leaf vegetable for its fresh, tart, bitter, and peppery flavor. Other common names include garden rocket (in Britain, Australia, South Africa, Ireland, and New Zealand), and eruca. It is also called "ruchetta," "rucola," "rucoli," "rugula," " colewort," and "roquette." ''Eruca sativa'', which is widely popular as a salad vegetable, is a species of '' Eruca'' native to the Mediterranean region, from Morocco and Portugal in the west to Syria, Lebanon, Palestine, Egypt and Turkey in the east.Med-Checklist''Eruca sativa''./ref>Blamey, M. & Grey-Wilson, C. (1989). ''Flora of Britain and Northern Europe''. . It is sometimes conflated with ''Diplotaxis tenuifolia'', known as "perennial wall rocket," another plant of the family Brassicaceae ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Agricultural And Food Chemistry
The ''Journal of Agricultural and Food Chemistry'' is a weekly peer-reviewed scientific journal established in 1953 by the American Chemical Society. Since 2015, Thomas Hofmann (Technical University of Munich) has been the editor-in-chief. The journal covers research dealing with the chemistry and biochemistry of agriculture and food including work with chemistry and/or biochemistry as a major component combined with biological/sensory/nutritional/toxicological evaluation related to agriculture and/or food. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, ProQuest, PubMed, CABI, and the Science Citation Index Expanded. According to the ''Journal Citation Reports'', the ''Journal of Agricultural and Food Chemistry'' has a 2015 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allium Fistulosum
''Allium fistulosum'', the Welsh onion, also commonly called bunching onion, long green onion, Japanese bunching onion, and spring onion, is a species of perennial plant, often considered to be a kind of scallion. The species is very similar in taste and odor to the related common onion, ''Allium cepa'', and hybrids between the two (tree onions) exist. ''A. fistulosum'', however, does not develop bulbs, and possesses hollow leaves (''fistulosum'' means "hollow") and scapes. Larger varieties of ''A. fistulosum'', such as the Japanese ''negi'', resemble the leek, whilst smaller varieties resemble chives. ''A. fistulosum'' can multiply by forming perennial evergreen clumps. It is also grown in a bunch as an ornamental plant. Names The common name "Welsh onion" does not refer to Wales but derives from a near obsolete use of "Welsh" in the sense "foreign, non-native", as the species is native to China, though cultivated in many places and naturalized in scattered locations i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing ''n''-butane to crude maleic anhydride, followed by catalytic hydrogenation. A third major industrial route entails hydroformylation of allyl alcohol follow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Letters
''Organic Letters'' is a biweekly peer-reviewed scientific journal covering research in organic chemistry. It was established in 1999 and is published by the American Chemical Society. In 2014, the journal moved to a hybrid open access publishing model. The founding editor-in-chief was Amos Smith. Since 2019, Erick M. Carreira serves as the editor-in-chief. The journal is abstracted and indexed in: the Science Citation Index Expanded, Scopus, Academic Search Premier, BIOSIS Previews, Chemical Abstracts Service, EMBASE, and MEDLINE MEDLINE (Medical Literature Analysis and Retrieval System Online, or MEDLARS Online) is a bibliographic database of life sciences and biomedical information. It includes bibliographic information for articles from academic journals covering medic .... References External links * American Chemical Society academic journals Biweekly journals Organic chemistry journals Publications established in 1999 English-language journals {{chem-j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allium Cepa
An onion (''Allium cepa'' L., from Latin ''cepa'' meaning "onion"), also known as the bulb onion or common onion, is a vegetable that is the most widely cultivated species of the genus ''Allium''. The shallot is a botanical variety of the onion which was classified as a separate species until 2010. Its close relatives include garlic, scallion, leek, and chive. This genus also contains several other species variously referred to as onions and cultivated for food, such as the Japanese bunching onion (''Allium fistulosum''), the tree onion (''A.'' × ''proliferum''), and the Canada onion (''Allium canadense''). The name ''wild onion'' is applied to a number of ''Allium'' species, but ''A. cepa'' is exclusively known from cultivation. Its ancestral wild original form is not known, although escapes from cultivation have become established in some regions. The onion is most frequently a biennial or a perennial plant, but is usually treated as an annual and harvested in its fir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allium Schoenoprasum
Chives, scientific name ''Allium schoenoprasum'', is a species of flowering plant in the family Amaryllidaceae that produces edible leaves and flowers. Their close relatives include the common onions, garlic, shallot, leek, scallion, and Chinese onion. A perennial plant, it is widespread in nature across much of Europe, Asia, and North America. ''A. schoenoprasum'' is the only species of ''Allium'' native to both the New and the Old Worlds.Ernest Small James Cullen, Sabina G. Knees, H. Suzanne Cubey (Editors) Chives are a commonly used herb and can be found in grocery stores or grown in home gardens. In culinary use, the green stalks ( scapes) and the unopened, immature flower buds are diced and used as an ingredient for omelettes, fish, potatoes, soups, and many other dishes. The edible flowers can be used in salads. Chives have insect-repelling properties that can be used in gardens to control pests. The plant provides a great deal of nectar for pollinators. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |