bottromycin on:
[Wikipedia]
[Google]
[Amazon]
Bottromycin is a macrocyclic
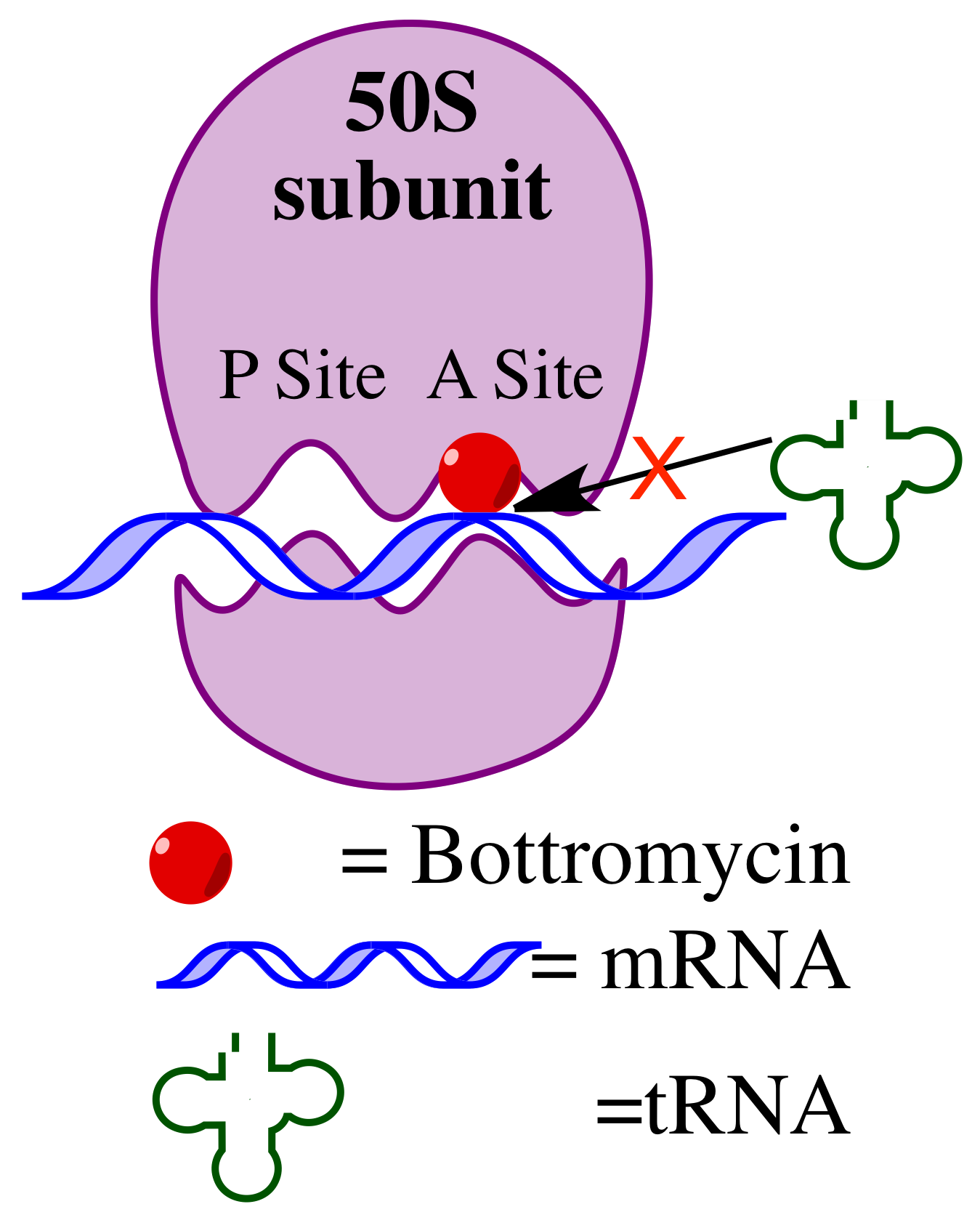 The mechanism of action of bottromycin was confirmed nearly 20 years following the discovery of bottromycin. Bottromycin functions as an antibiotic through inhibition of protein synthesis. It blocks aminoacyl ''t''RNA binding to the ribosome by binding to the A site of the 50s subunit. This results in release of aminoacyl ''t''RNA from the ribosome and premature termination of protein synthesis. A comparison of other antibiotics known to bind to the A site of the ribosome, including micrococcin,
The mechanism of action of bottromycin was confirmed nearly 20 years following the discovery of bottromycin. Bottromycin functions as an antibiotic through inhibition of protein synthesis. It blocks aminoacyl ''t''RNA binding to the ribosome by binding to the A site of the 50s subunit. This results in release of aminoacyl ''t''RNA from the ribosome and premature termination of protein synthesis. A comparison of other antibiotics known to bind to the A site of the ribosome, including micrococcin,
 These early structural studies were not followed up until recent years with the renewed interest in bottromycin. The structure was confirmed in the 1980s and 1990s to be a cyclic iminopeptide based on NMR studies, with a linear side chain connected to the macrocycle via an amidine linkage.
Its absolute stereochemistry, however, was not characterized until 2009. Stereochemistry at carbon 18 and 25 was proposed by comparing predicted conformers obtained using
These early structural studies were not followed up until recent years with the renewed interest in bottromycin. The structure was confirmed in the 1980s and 1990s to be a cyclic iminopeptide based on NMR studies, with a linear side chain connected to the macrocycle via an amidine linkage.
Its absolute stereochemistry, however, was not characterized until 2009. Stereochemistry at carbon 18 and 25 was proposed by comparing predicted conformers obtained using
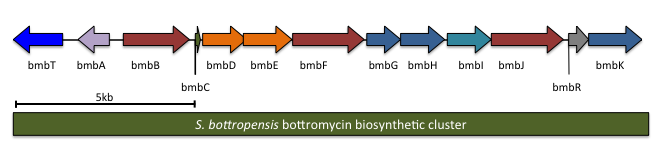 The complete biosynthetic gene cluster for bottromycin has been identified. It is predicted to contain 13 genes, including the precursor peptide (notation will follow Crone and colleagues; other studies had similar results). One of the genes in the cluster, ''btmL'', is proposed to be a
The complete biosynthetic gene cluster for bottromycin has been identified. It is predicted to contain 13 genes, including the precursor peptide (notation will follow Crone and colleagues; other studies had similar results). One of the genes in the cluster, ''btmL'', is proposed to be a 
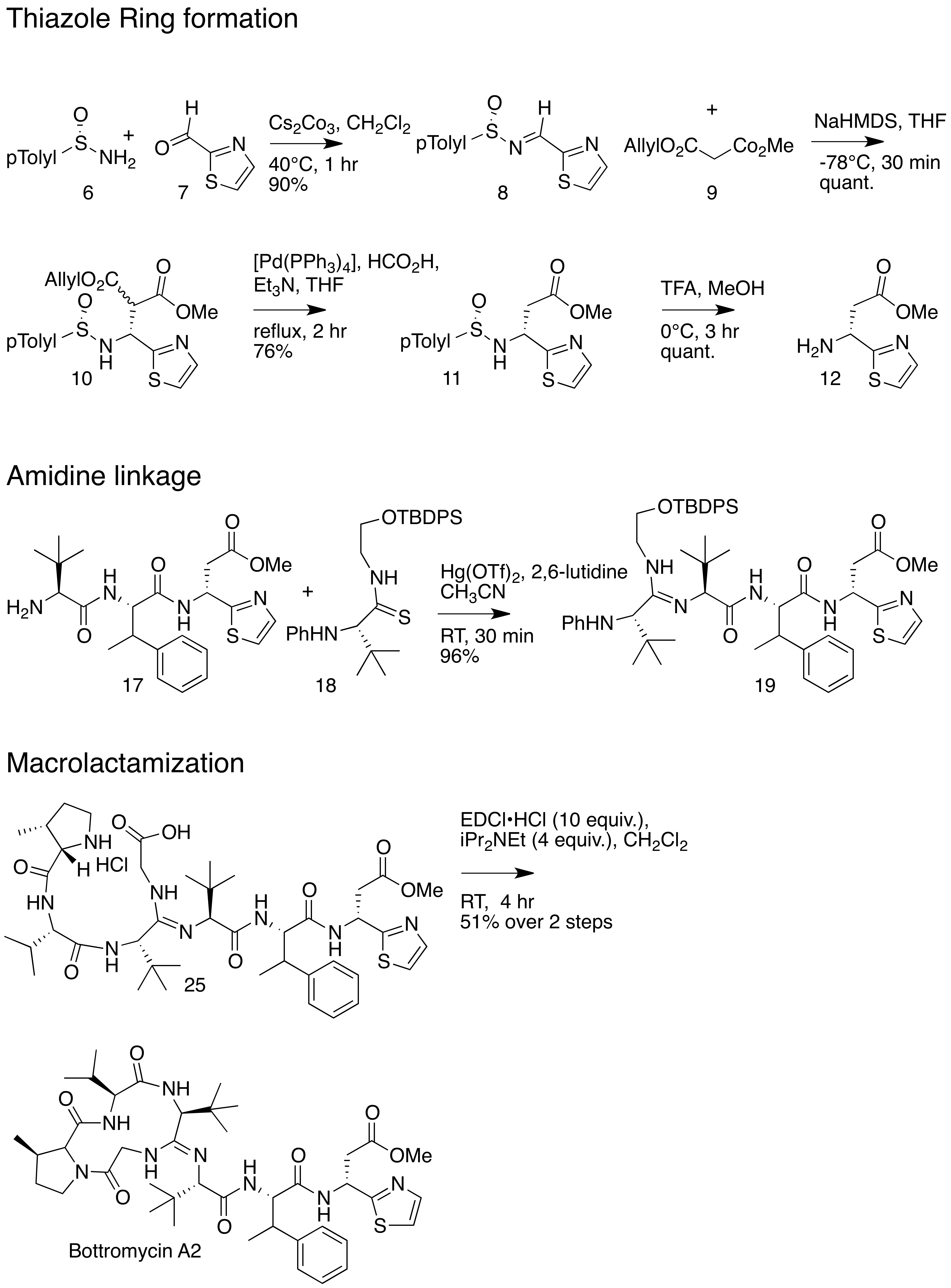 The total synthesis of bottromycin was accomplished in 2009. The synthesis was achieved in 17 steps. Although bottromycin is a peptide-based natural product, it contains an unusual macrocycle and thiazole heterocycle, so that the total synthesis could not be accomplished using traditional solid-phase peptide synthesis. The synthesis was accomplished using a combination of peptide coupling and other methods. To obtain the primary thia-β-Ala-OMe intermediate, a sequence of condensation,
The total synthesis of bottromycin was accomplished in 2009. The synthesis was achieved in 17 steps. Although bottromycin is a peptide-based natural product, it contains an unusual macrocycle and thiazole heterocycle, so that the total synthesis could not be accomplished using traditional solid-phase peptide synthesis. The synthesis was accomplished using a combination of peptide coupling and other methods. To obtain the primary thia-β-Ala-OMe intermediate, a sequence of condensation,  In 2012, an alternative synthesis of the bottromycin macrocyclic ring system and amidine linkage was reported. The synthesis was achieved in 10 steps. Unlike the previous synthesis, Ackerman and colleagues synthesized a linear peptide and achieved intramolecular amidine formation using an ''S''-methylated endothiopeptide. The endothiopeptide was obtained by a thio-Ugi reaction. The resulting macrocycle was obtained as a racemic mixture at the amidine linkage. The full synthetic scheme may be viewed under the collapsed synthetic scheme link.
In 2012, an alternative synthesis of the bottromycin macrocyclic ring system and amidine linkage was reported. The synthesis was achieved in 10 steps. Unlike the previous synthesis, Ackerman and colleagues synthesized a linear peptide and achieved intramolecular amidine formation using an ''S''-methylated endothiopeptide. The endothiopeptide was obtained by a thio-Ugi reaction. The resulting macrocycle was obtained as a racemic mixture at the amidine linkage. The full synthetic scheme may be viewed under the collapsed synthetic scheme link.
 Following the total synthesis of bottromycin, Kobayashi and colleagues synthesized a series of bottromycin derivatives and evaluated their anti-MRSA and anti-VRE activity. Only derivatives of the methyl ester moiety were explored, as they found that the methyl ester was both important for antibacterial activity and unstable in blood plasma. A series of seventeen derivatives were synthesized, with derivatives falling into three general categories:
Following the total synthesis of bottromycin, Kobayashi and colleagues synthesized a series of bottromycin derivatives and evaluated their anti-MRSA and anti-VRE activity. Only derivatives of the methyl ester moiety were explored, as they found that the methyl ester was both important for antibacterial activity and unstable in blood plasma. A series of seventeen derivatives were synthesized, with derivatives falling into three general categories:
peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
with antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
activity. It was first discovered in 1957 as a natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
isolated from ''Streptomyces bottropensis
''Streptomyces bottropensis'' is a bacterium species from the genus '' Streptomyces'' which has been isolated from soil.Deutsche Sammlung von Mikroorganismen und Zellkulturenbr>/ref> ''Streptomyces bottropensis'' produces bottromycin, dunaimyci ...
''. It has been shown to inhibit methicillin-resistant ''Staphylococcus aureus'' (MRSA
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. ...
) and vancomycin-resistant Enterococci ( VRE) among other Gram-positive bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
Gram-positive bact ...
and mycoplasma
''Mycoplasma'' is a genus of bacteria that, like the other members of the class ''Mollicutes'', lack a cell wall around their cell membranes. Peptidoglycan (murein) is absent. This characteristic makes them naturally resistant to antibiotics ...
. Bottromycin is structurally distinct from both vancomycin
Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is recommended intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, ...
, a glycopeptide antibiotic
Glycopeptide antibiotics are a class of drugs of microbial origin that are composed of glycosylated cyclic or polycyclic nonribosomal peptides. Significant glycopeptide antibiotics include the anti-infective antibiotics vancomycin, teicoplanin, ...
, and methicillin
Methicillin (USAN), also known as meticillin (INN), is a narrow-spectrum β-lactam antibiotic of the penicillin class.
Methicillin was discovered in 1960.
Medical uses
Compared to other penicillins that face antimicrobial resistance due ...
, a beta-lactam antibiotic
β-lactam antibiotics (beta-lactam antibiotics) are antibiotics that contain a beta-lactam ring in their chemical
structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and ...
.
Bottromycin binds to the A site of the ribosome
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to ...
and blocks the binding of aminoacyl-''t''RNA, therefore inhibiting bacterial protein synthesis. Although bottromycin exhibits antibacterial activity ''in vitro'', it has not yet been developed as a clinical antibiotic, potentially due to its poor stability in blood plasma. To increase its stability ''in vivo'', some bottromycin derivatives have been explored.
The structure of bottromycin contains a macrocyclic amidine
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2.
Examples of amidines includ ...
as well as a thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular for ...
ring. The absolute stereochemistry at several chiral centers has been determined as of 2009. In 2012, a three-dimensional solution structure of bottromycin was published. The solution structure revealed that several methyl groups are on the same face of the structure.
Bottromycin falls within the ribosomally synthesized and post-translationally modified peptide class of natural product.
History
Bottromycin was first isolated from ''Streptomyces bottropensis'' in 1957. It has since been identified in at least two other members of the genus ''Streptomyces
''Streptomyces'' is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of ''Streptomyces'' bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, ...
''; members of ''Streptomyces'' are known to be prolific producers of secondary metabolites. Bottromycin has a unique structure, consisting of the macrocyclic amidine linkage and four β-methylated amino acids. Bottromycin blocks aminoacyl ''t''RNA binding to the ribosome by binding to the A site of the 50s subunit. Although bottromycin was discovered over 50 years ago, there was a lack of research following initial studies on bottromycin until recent years. The lack of research is potentially a result of bottromycin's low stability in blood plasma. However, the unique structure and mode of action have recently made bottromycin a more target for drug development, especially given the rise of antibiotic resistance.
Mechanism of action
 The mechanism of action of bottromycin was confirmed nearly 20 years following the discovery of bottromycin. Bottromycin functions as an antibiotic through inhibition of protein synthesis. It blocks aminoacyl ''t''RNA binding to the ribosome by binding to the A site of the 50s subunit. This results in release of aminoacyl ''t''RNA from the ribosome and premature termination of protein synthesis. A comparison of other antibiotics known to bind to the A site of the ribosome, including micrococcin,
The mechanism of action of bottromycin was confirmed nearly 20 years following the discovery of bottromycin. Bottromycin functions as an antibiotic through inhibition of protein synthesis. It blocks aminoacyl ''t''RNA binding to the ribosome by binding to the A site of the 50s subunit. This results in release of aminoacyl ''t''RNA from the ribosome and premature termination of protein synthesis. A comparison of other antibiotics known to bind to the A site of the ribosome, including micrococcin, tetracycline
Tetracycline, sold under various brand names, is an oral antibiotic in the tetracyclines family of medications, used to treat a number of infections, including Acne vulgaris, acne, cholera, brucellosis, plague (disease), plague, malaria, and sy ...
, streptomycin
Streptomycin is an antibiotic medication used to treat a number of bacterial infections, including tuberculosis, ''Mycobacterium avium'' complex, endocarditis, brucellosis, ''Burkholderia'' infection, plague, tularemia, and rat bite fever. Fo ...
, and chloramphenicol
Chloramphenicol is an antibiotic useful for the treatment of a number of bacterial infections. This includes use as an eye ointment to treat conjunctivitis. By mouth or by injection into a vein, it is used to treat meningitis, plague, cholera, a ...
, suggested that only bottromycin and chloramphenicol caused release of aminoacyl ''t''RNA from the ribosome. Of those antibiotics, only micrococcin is also a macrocyclic peptide.
Structure determination
Bottromycin is produced naturally as a series of products differing in methylation patterns. All products contain valine and phenylalanine methylation. Bottromycin A2 is singly methylated on proline, bottromycin B lacks methylation on proline, and bottromycin C contains a doubly methylated proline. A partial structure of bottromycin was reported shortly after the initial discovery of bottromycin. The first structural studies relied on traditional methods of analysis. Itspeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
-like structure, including the presence of glycine and valine, was first suggested by a combination of acidic hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
, acetylation
:
In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply '' acetates''. Deacetylation is the oppo ...
, ninhydrin
Ninhydrin (2,2-dihydroxyindane-1,3-dione) is an organic compound with the formula C6H4(CO)2C(OH)2. It is used to detect ammonia and amines. Upon reaction with these amines, ninhydrin gets converted into deep blue or purple derivatives, which are ...
staining, and paper chromatography
Paper chromatography is an analytical method used to separate coloured chemicals or substances. It is now primarily used as a teaching tool, having been replaced in the laboratory by other chromatography methods such as thin-layer chromatography ...
, among other experiments. The presence of a thiazole ring, along with an adjacent β-methylated phenylalanine, was established by ninhydrin staining, potassium permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution.
Potassium permanganate is widely used in the c ...
oxidation, and comparison to synthetic standards. A methyl ester substituent was reported in 1958. The same study also reported that the Kunz hydrolysis product lacking a methyl ester was biologically inactive. Nakamura and colleagues later reported that bottromycin contained tert-leucine and ''cis''-3-methylproline. They also proposed a linear iminohexapeptide structure.
 These early structural studies were not followed up until recent years with the renewed interest in bottromycin. The structure was confirmed in the 1980s and 1990s to be a cyclic iminopeptide based on NMR studies, with a linear side chain connected to the macrocycle via an amidine linkage.
Its absolute stereochemistry, however, was not characterized until 2009. Stereochemistry at carbon 18 and 25 was proposed by comparing predicted conformers obtained using
These early structural studies were not followed up until recent years with the renewed interest in bottromycin. The structure was confirmed in the 1980s and 1990s to be a cyclic iminopeptide based on NMR studies, with a linear side chain connected to the macrocycle via an amidine linkage.
Its absolute stereochemistry, however, was not characterized until 2009. Stereochemistry at carbon 18 and 25 was proposed by comparing predicted conformers obtained using molecular dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the ...
to experimental constraints obtained through NMR experiments. Stereochemistry at carbon 43 was confirmed by comparing 1H NMR of authentic hydrolysis product to a chemically synthesized sample of the same fragment. Finally, optical rotation, 1H NMR, and HRMS experiments of chemically synthesized bottromycin matched that of biologically produced bottromycin.
The three-dimensional solution structure of bottromycin A2 was solved by NMR in 2012. The overall structure was obtained with good resolution (RMSD 0.74±0.59 Å), with a RMSD of 0.09±0.06 Å for the macrocycle. In this study, it was proposed that the methylated proline residue contributed to the restricted conformation of the macrocycle. The methylated proline and β-OMe alanine residues were found to be on the same face of bottroymycin A2 and it was suggested that this characteristic contributed to binding of bottromycin to the ribosomal A site.
Biosynthesis
The production of bottromycin by ''S. bottropensis'' and ''S. scabies'', as well as the production of a bottromycin analog termed bottromycin D, has been studied. It was independently confirmed in 2012 by multiple groups that bottromycin is produced as a ribosomal peptide natural product that it subsequently post-translationally modified. Before this, it was unclear whether bottromycin was produced bynonribosomal peptide Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacter ...
synthetase machinery (NRPS). The presence of amino acids other than the 20 proteinogenic amino acids is often a feature of NRPS products because NRPS machinery can directly incorporate other amino acids, among other chemical building blocks. Ribosomal peptide synthesis, which is the same machinery that produces all proteins found in the cell, is limited to the 20 proteinogenic amino acids. However, bottromycin was found to be a highly modified ribosomal peptide by a combination of genome mining and gene deletion studies.
In ribosomal peptide synthesis, the final product results from modifications to a linear peptide starting material translated by the ribosome from an mRNA transcript. In ''S. scabies'' the precursor peptide, termed BtmD, is a 44-amino acid peptide. The precursor peptide is termed BmbC in ''S. bottropensis''. The amino acids forming the bottromycin core are residues 2-9 in BtmD: Gly-Pro-Val-Val-Val-Phe-Asp-Cys. In bottromycin D, the sequence is Gly-Pro-Ala-Val-Val-Phe-Asp-Cys, and the precursor peptide is termed BstA. BstA shares high sequence homology with BtmD in the follower peptide region. Unlike other ribosomal peptide natural products, which are normally synthesized with a leader peptide that is cleaved, bottromycin is synthesized with a follower peptide. The presence of a follower peptide was identified by bioinformatic analysis of the bottromycin biosynthetic cluster.
 The complete biosynthetic gene cluster for bottromycin has been identified. It is predicted to contain 13 genes, including the precursor peptide (notation will follow Crone and colleagues; other studies had similar results). One of the genes in the cluster, ''btmL'', is proposed to be a
The complete biosynthetic gene cluster for bottromycin has been identified. It is predicted to contain 13 genes, including the precursor peptide (notation will follow Crone and colleagues; other studies had similar results). One of the genes in the cluster, ''btmL'', is proposed to be a transcriptional regulator
In molecular biology and genetics, transcriptional regulation is the means by which a cell regulates the conversion of DNA to RNA ( transcription), thereby orchestrating gene activity. A single gene can be regulated in a range of ways, from a ...
. Another gene, ''btmA'', is proposed to export bottromycin. The remaining ten genes are expected to modify the precursor peptide ''btmD'' from a linear peptide to the final macrocyclic product.
A biosynthetic pathway
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
has been hypothesized based on proposed gene functions (see figure). ''btmM'', with homology to Zn+2 aminopeptidases, is predicted to cleave the N-terminal methionine residue, which is not present in the bottromycin final product. ''btmE'' and ''btmF'' both contain YcaO
YcaO is a protein found in bacteria which is involved in the synthesis of thiazole/oxazole modified microcin antibiotics, such as bottromycin. YcaO performs ATP dependent cyclodehydration to form the oxazole and thiazole moieties of the microcin. ...
-like domains. It is believed that one Although it is unclear which enzyme is responsible for which step, it is hypothesized that one catalyzes macrocyclic amidine formation while the other catalyzes thiazoline formation. ''btmJ'', encoding an enzyme with cytochrome P450 homology, may oxidize the thiazoline to the thiazole. ''btmH'' or ''btmI'' both have homology to hydrolytic enzymes (α/β hydrolase and metallo-dependent hydrolase, respectively) may catalyze follower peptide hydrolysis. An alternative proposed role for ''btmH'' or ''btmI'' is to function as a cyclodehydratase in macrocyclization. Gene deletion studies failed to elucidate the function of other proteins within the cluster.

Methyltransferases in the biosynthetic cluster
Bioinformatic analysis identified four methyltransferases within the cluster. Bioinformatics suggest that ''btmB'', is an ''O''-methyltransferase, while the other three, ''btmC'', ''G'' and ''K'', are radical ''S''-adenosyl methionine (SAM) methyltransferases. The radical SAM methyltransferases are believed to β-methylate amino acid residues within the precursor peptide. ''btmC'' is believed to methylate phenylalanine, ''btmG'' is believed to methylate both valines, and ''btmK'' is believed to methylate proline based on gene deletion studies. The three putative radical SAM methyltransferases encoded within the pathway are interesting for both mechanistic and biosynthetic reasons. Radical SAM methyltransferases are likely to methylate substrates by an unusual mechanism. Biosynthetically, β-methylations of amino acids are highly unusual in natural products. Polytheonamide B, a peptide natural product produced by a marine symbiont, is the only other structurally characterized example of direct β-methylation of a peptide natural product. The proposed methyl transfer from a SAM-utilizing enzyme was supported by earlier feeding studies with labeled methionine; labeled methionine is used because methionine is converted into SAM within cells. Even further, this study used stereospecifically labeled methionine ( ethyl-(2H-3H)(2''S'', methyl-''R'')-methionine) to show that methylation occurred with a net retention of stereochemistry at the methyl group. The author speculated that net retention indicated a radical mechanism with a B12 intermediate. Radical transfer with aCobalamin
Vitamin B12, also known as cobalamin, is a water-soluble vitamin involved in metabolism. It is one of eight B vitamins. It is required by animals, which use it as a cofactor in DNA synthesis, in both fatty acid and amino acid metabolism. It ...
B12 cofactor and SAM has been shown with the few characterized radical SAM methyltransferases. Although the evidence points to radical β-methylation during bottromycin biosynthesis, it remains to be seen whether bioinformatic hypothesis and feeding studies will be supported by ''in vitro'' activity assays.
The Val3Ala substitution in bottromycin D does not change the β-methylation pattern between bottromycin A2 and D because Val3 is the only valine ''not'' methylated in bottromycin A2. As such, there are still three predicted radical SAM dependent enzymes in the bottromycin D biosynthetic cluster: ''bstC'', ''bstF'', and ''bstJ''.
As of 2013, all published biosynthetic studies have been bioinformatic or cell-based. No biochemical assays directly demonstrating protein function have yet been published. It is likely that ''in vitro'' mechanistic studies to better elucidate the biosynthetic pathway will be forthcoming.
Total synthesis
 The total synthesis of bottromycin was accomplished in 2009. The synthesis was achieved in 17 steps. Although bottromycin is a peptide-based natural product, it contains an unusual macrocycle and thiazole heterocycle, so that the total synthesis could not be accomplished using traditional solid-phase peptide synthesis. The synthesis was accomplished using a combination of peptide coupling and other methods. To obtain the primary thia-β-Ala-OMe intermediate, a sequence of condensation,
The total synthesis of bottromycin was accomplished in 2009. The synthesis was achieved in 17 steps. Although bottromycin is a peptide-based natural product, it contains an unusual macrocycle and thiazole heterocycle, so that the total synthesis could not be accomplished using traditional solid-phase peptide synthesis. The synthesis was accomplished using a combination of peptide coupling and other methods. To obtain the primary thia-β-Ala-OMe intermediate, a sequence of condensation, Mannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia (). ...
, and palladium-catalyzed decarboxylation steps were performed. This intermediate was prepared stereoselectively. To obtain the amidine linkage, a tripeptide intermediate was coupled to a phthaloyl-protected thioamide via mercury-mediated condensation using mercury (II) trifluoromethanesulfonate (Hg(OTf)2) to yield a branched amidine intermediate. To obtain the final product macrocycle, macrolactamization of the amidine-containing intermediate was required. Macrolactamization was performed with 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, EDAC or EDCI) is a water-soluble carbodiimide usually handled as the hydrochloride. It is typically employed in the 4.0-6.0 pH range. It is generally used as a carboxyl activating agent for the ...
(EDCI) and iPr2NEt yielded the final product, bottromycin A2. To confirm that the synthesized bottromycin A2 had the same stereochemistry as natural bottromycin A2, the product was studied by optical rotation
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
, 1H and 13C NMR, IR, and HRMS. The data was found to match that of isolated bottromycin A2. Further, the synthetic sample of bottromycin was also found to have antibacterial activity against both MRSA and VRE, although quantitative data was not reported. A full rendering of the synthetic scheme may be seen under the collapsed synthetic scheme link.
 In 2012, an alternative synthesis of the bottromycin macrocyclic ring system and amidine linkage was reported. The synthesis was achieved in 10 steps. Unlike the previous synthesis, Ackerman and colleagues synthesized a linear peptide and achieved intramolecular amidine formation using an ''S''-methylated endothiopeptide. The endothiopeptide was obtained by a thio-Ugi reaction. The resulting macrocycle was obtained as a racemic mixture at the amidine linkage. The full synthetic scheme may be viewed under the collapsed synthetic scheme link.
In 2012, an alternative synthesis of the bottromycin macrocyclic ring system and amidine linkage was reported. The synthesis was achieved in 10 steps. Unlike the previous synthesis, Ackerman and colleagues synthesized a linear peptide and achieved intramolecular amidine formation using an ''S''-methylated endothiopeptide. The endothiopeptide was obtained by a thio-Ugi reaction. The resulting macrocycle was obtained as a racemic mixture at the amidine linkage. The full synthetic scheme may be viewed under the collapsed synthetic scheme link.
Derivatives
 Following the total synthesis of bottromycin, Kobayashi and colleagues synthesized a series of bottromycin derivatives and evaluated their anti-MRSA and anti-VRE activity. Only derivatives of the methyl ester moiety were explored, as they found that the methyl ester was both important for antibacterial activity and unstable in blood plasma. A series of seventeen derivatives were synthesized, with derivatives falling into three general categories:
Following the total synthesis of bottromycin, Kobayashi and colleagues synthesized a series of bottromycin derivatives and evaluated their anti-MRSA and anti-VRE activity. Only derivatives of the methyl ester moiety were explored, as they found that the methyl ester was both important for antibacterial activity and unstable in blood plasma. A series of seventeen derivatives were synthesized, with derivatives falling into three general categories: amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
derivatives, urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important r ...
derivatives, and ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
derivatives. All analogs except the carboxylic acid and hydrazide analogs were derivatized from isolated bottromycin A2 using an activated azide ester. The derivatives were tested against six Gram-positive bacterial strains: ''Staphylococcus aureus
''Staphylococcus aureus'' is a Gram-positive spherically shaped bacterium, a member of the Bacillota, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive ...
'' FDA209P, ''S. aureus'' Smith, MRSA HH-1, MRSA 92-1191, ''Enterococcus faecalis
''Enterococcus faecalis'' – formerly classified as part of the group D ''Streptococcus'' system – is a Gram-positive, commensal bacterium inhabiting the gastrointestinal tracts of humans. Like other species in the genus ''Enterococcus'', ''E ...
'' NCTC12201, and ''E. faecalis'' NCTC12203 (both VRE).
Bottromycin A2 had low micromolar activity against all the strains tested, ranging from an MIC of 0.5 μg/mL in ''E. faecalis'' NCTC12203 to 2 μg/mL in MRSA HH-1. The amide and urea derivative families were found to have weaker antibacterial activity than bottromycin A2 against ''S. aureus'', MRSA, and VRE. The MIC values for the amide and urea derivatives were generally four times greater than those for bottromycin A2. They were, however, significantly more stable in mouse plasma than bottromycin A2. Bottromycin A2 completely degraded in mouse plasma after 10 minutes and exhibited 0% residual activity after exposure to rat serum. Only one derivative had lower than 50% residual activity. In contrast, many derivatives retained a significant percentage of residual anti-MRSA activity following exposure to serum. Thioester intermediates to the ketone derivatives were found to be unstable, exhibiting 0% residual activity, although they had improved antibacterial activity, exhibiting sub-micromolar MIC values. The propyl ketone was found to be the most promising derivative of all the analogs obtained, both exhibiting antibacterial activity against the bacterial strains tested and stability in plasma, retaining 100% residual activity. The MIC values obtained for the propyl derivative were the same as those found for bottromycin A2 except in the case of NCTC12201, which had an MIC of 2 μg/mL for the derivative and an MIC of 1 μg/mL for bottromycin A2. A summary of MIC values for tested bacterial strains is shown below.
Even the least active bottromycin derivatives exhibited greater anti-VRE activity than vancomycin, which was used as a control antibiotic in this study. The propyl derivative and bottromycin A2 had similar antimicrobial activity to linezolid
Linezolid is an antibiotic used for the treatment of infections caused by Gram-positive bacteria that are resistant to other antibiotics. Linezolid is active against most Gram-positive bacteria that cause disease, including streptococci, van ...
, a synthetic antibiotic active against Gram-positive bacteria including MRSA and VRE, across all the bacterial strains studied. Overall, the results of this study suggested that further modifications of bottromycin may lead to a more stable, effective antibiotic.
A natural derivative of bottromycin, bottromycin D, has also been identified. It is produced in a marine ''Streptomyces'' species, strain WMMB272. Although the methyl ester is still present in bottromycin D, one of the macrocyclic valines is mutated to an alanine. The minimum inhibitory concentration In microbiology, the minimum inhibitory concentration (MIC) is the lowest concentration of a chemical, usually a drug, which prevents visible growth of a bacterium or bacteria. MIC depends on the microorganism, the affected human being (in vivo onl ...
(MIC) for bottromycin D was determined and found to be only slightly less active than bottromycin A2 (2 μg/mL for bottromycin D vs. 1 μg/mL for bottromycin A2). The authors postulated that greater conformational flexibility of bottromycin D may be responsible for its lower activity.
No further antibacterial studies of synthetic or biosynthetic bottromycin derivatives have been reported in the literature as of 2013. The search for efficacious analogs will be enabled by bottromycin’s status as a ribosomal peptide. Analogs may be explored biosynthetically by changing the sequence of the precursor peptide; a change in amino acid sequence will lead directly to a modified bottromycin structure.
Clinical potential
As of 2013, bottromycin has not been approved for any clinical applications, nor has it been tested in humans. The ''in vivo'' stability of bottromycin must be improved before it can be considered as a drug candidate. Work by Kobayashi and colleagues has already begun to address this issue, but more work may be in progress. The need to find new antibiotics to combat antibiotic resistance means that biologic and synthetic interest in bottromycin will likely continue. A combination of biologic and synthetic techniques may yield both an efficacious and stable bottromycin analog for development as a potential drug candidate.See also
*Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
*''Streptomyces
''Streptomyces'' is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of ''Streptomyces'' bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, ...
''
*Secondary metabolite
Secondary metabolites, also called specialised metabolites, toxins, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, fungi, animals, or plants, which are not directly involved in the norm ...
*Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
*MRSA
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. ...
*Vancomycin-resistant Enterococcus
Vancomycin-resistant ''Enterococcus'', or vancomycin-resistant enterococci (VRE), are bacterial strains of the genus ''Enterococcus'' that are resistant to the antibiotic vancomycin.
Mechanism of acquired resistance
Six different types of vanc ...
References
{{Reflist Peptides Antibiotics Streptomyces 2-Thiazolyl compounds Total synthesis