|
Radical SAM
Radical SAM is a designation for a superfamily of enzymes that use a +_cluster.html" ;"title="Fe-4Ssup>+ cluster">Fe-4Ssup>+ cluster to reductively cleave ''S''-adenosyl-L-methionine (SAM) to generate a radical, usually a 5′- deoxyadenosyl radical (5'-dAdo), as a critical intermediate. These enzymes utilize this radical intermediate to perform diverse transformations, often to functionalize unactivated C-H bonds. Radical SAM enzymes are involved in cofactor biosynthesis, enzyme activation, peptide modification, post-transcriptional and post-translational modifications, metalloprotein cluster formation, tRNA modification, lipid metabolism, biosynthesis of antibiotics and natural products etc. The vast majority of known radical SAM enzymes belong to the radical SAM superfamily, and have a cysteine-rich motif that matches or resembles CxxxCxxC. rSAMs comprise the largest superfamily of metal-containing enzymes. History and mechanism As of 2001, 645 unique radical SAM enzymes h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4Fe-4S
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine
Adenosine (symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9- glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias. Adenosyl (abbreviated Ado or 5'-dAdo) is the chemical group formed by removal of the 5′-hydroxy (OH) group. It is found in adenosylcobalamin (an active form of vitamin B12) and as a radical in radical SAM enzymes. Medical uses Supraventricular tachycardia In individuals with supraventricular tachycardia (SVT), adenosine is used to help identify and convert t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salmonella Enterica Subsp
''Salmonella'' is a genus of rod-shaped (bacillus) Gram-negative bacteria of the family Enterobacteriaceae. The two species of ''Salmonella'' are '' Salmonella enterica'' and '' Salmonella bongori''. ''S. enterica'' is the type species and is further divided into six subspecies that include over 2,600 serotypes. ''Salmonella'' was named after Daniel Elmer Salmon (1850–1914), an American veterinary surgeon. ''Salmonella'' species are non-spore-forming, predominantly motile enterobacteria with cell diameters between about 0.7 and 1.5 μm, lengths from 2 to 5 μm, and peritrichous flagella (all around the cell body, allowing them to move). They are chemotrophs, obtaining their energy from oxidation and reduction reactions, using organic sources. They are also facultative anaerobes, capable of generating ATP with oxygen ("aerobically") when it is available, or using other electron acceptors or fermentation ("anaerobically") when oxygen is not available. ''Salmonella'' s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiolation
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strongly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticodons
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino acid sequence of proteins. tRNAs genes from Bacteria are typically shorter (mean = 77.6 bp) than tRNAs from Archaea (mean = 83.1 bp) and eukaryotes (mean = 84.7 bp). The mature tRNA follows an opposite pattern with tRNAs from Bacteria being usually longer (median = 77.6 nt) than tRNAs from Archaea (median = 76.8 nt), with eukaryotes exhibiting the shortest mature tRNAs (median = 74.5 nt). Transfer RNA (tRNA) does this by carrying an amino acid to the protein synthesizing machinery of a cell called the ribosome. Complementation of a 3-nucleotide codon in a messenger RNA (mRNA) by a 3-nucleotide anticodon of the tRNA results in protein synthesis based on the mRNA code. As such, tRNAs are a necessary component of translation, the biological sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylthiotransferase
Methylthiotransferases are enzymes of the radical S-adenosyl methionine (radical SAM) superfamily. These enzymes catalyze the addition of a methylthio group to various biochemical compounds including tRNA and proteins. Methylthiotransferases are classified into one of four classes based on their substrates and mechanisms. All methylthiotransferases have been shown to contain two Fe-S clusters, one canonical cluster and one auxiliary cluster, that both function in the addition of the methylthio group to the substrate. Overview Methylthiotransferases, also known as MTTases, are a subset of the radical SAM enzyme superfamily. These enzymes catalyze the addition of a methylthio group to either a protein or tRNA substrate. Radical S-adenosylmethionine enzymes, otherwise known as radical SAM enzymes, are metalloproteins that cleave S-adenosyl-L-methionine into L-methionine and a 5'-deoxyadenosyl 5'-radical (5'-dA). 5'-dA is an intermediate in the reactions catalyzed by radical SAMs. 5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer R5,10-methylene-THF. As an intermediate in one-carbon metabolism, 5,10-CH2-THF interconverts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR) It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase. Selected functions Formaldehyde detoxification Methylenetetrahydrofolate is an intermediate in the detoxification of formaldehyde. Pyrimidine biosynthesis It is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate (dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP). The coenzyme is necessary for the biosynthesis of thymidine and is the C1-donor in the reactions catalyzed by TS and thymidyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane itself is mainly of theoretical interest but many of its derivatives are of commercial or biological significance. History Cyclopropane was discovered in 1881 by August Freund, who also proposed the correct structure for the substance in his first paper. Freund treated 1,3-dibromopropane with sodium, causing an intramolecular Wurtz reaction leading directly to cyclopropane. The yield of the reaction was improved by Gustavson in 1887 with the use of zinc instead of sodium. Cyclopropane had no commercial application until Henderson and Lucas discovered its anaesthetic properties in 1929; industrial production had begun by 1936. In modern anaesthetic practice, it has been superseded by other agents. Anaesthesia Cyclopropane was introduced into cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiopeptide
Thiopeptides (thiazolyl peptides) are a class of peptide antibiotics produced by bacteria. They have antibiotic activity against Gram-positive bacteria, but little or no activity against Gram-negative bacteria. Many of the members of this class show activity against methicillin-resistant ''Staphylococcus aureus'' (MRSA) and are therefore subjects of research interest. There are over 100 members of this class known. Chemical structure Thiopeptides are sulfur-rich macrocyclic peptides containing highly-modified amino acids. They are characterized by a nitrogen-containing six-membered ring (such as piperidine, dehydropiperidine, or pyridine) substituted with multiple thiazole rings and dehydroamino acids. A macrocylic ring serves as a scaffold for a tail that also incorporates modified amino acids often with azole rings, such as thiazoles, oxazoles, and thiazolines which are derived from serine, threonine, and cysteine residues. Examples Examples of thiopeptides include thiost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coproporphyrinogen III Oxidase
Coproporphyrinogen-III oxidase, mitochondrial (abbreviated as CPOX) is an enzyme that in humans is encoded by the ''CPOX'' gene. A genetic defect in the enzyme results in a reduced production of heme in animals. The medical condition associated with this enzyme defect is called hereditary coproporphyria. CPOX, the sixth enzyme of the haem biosynthetic pathway, converts coproporphyrinogen III to protoporphyrinogen IX through two sequential steps of oxidative decarboxylation. The activity of the CPOX enzyme, located in the mitochondrial membrane, is measured in lymphocytes. Function CPOX is an enzyme involved in the sixth step of porphyrin metabolism it catalyses the oxidative decarboxylation of coproporphyrinogen III to proto-porphyrinogen IX in the haem and chlorophyll biosynthetic pathways. The protein is a homodimer containing two internally bound iron atoms per molecule of native protein. The enzyme is active in the presence of molecular oxygen that acts as an electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
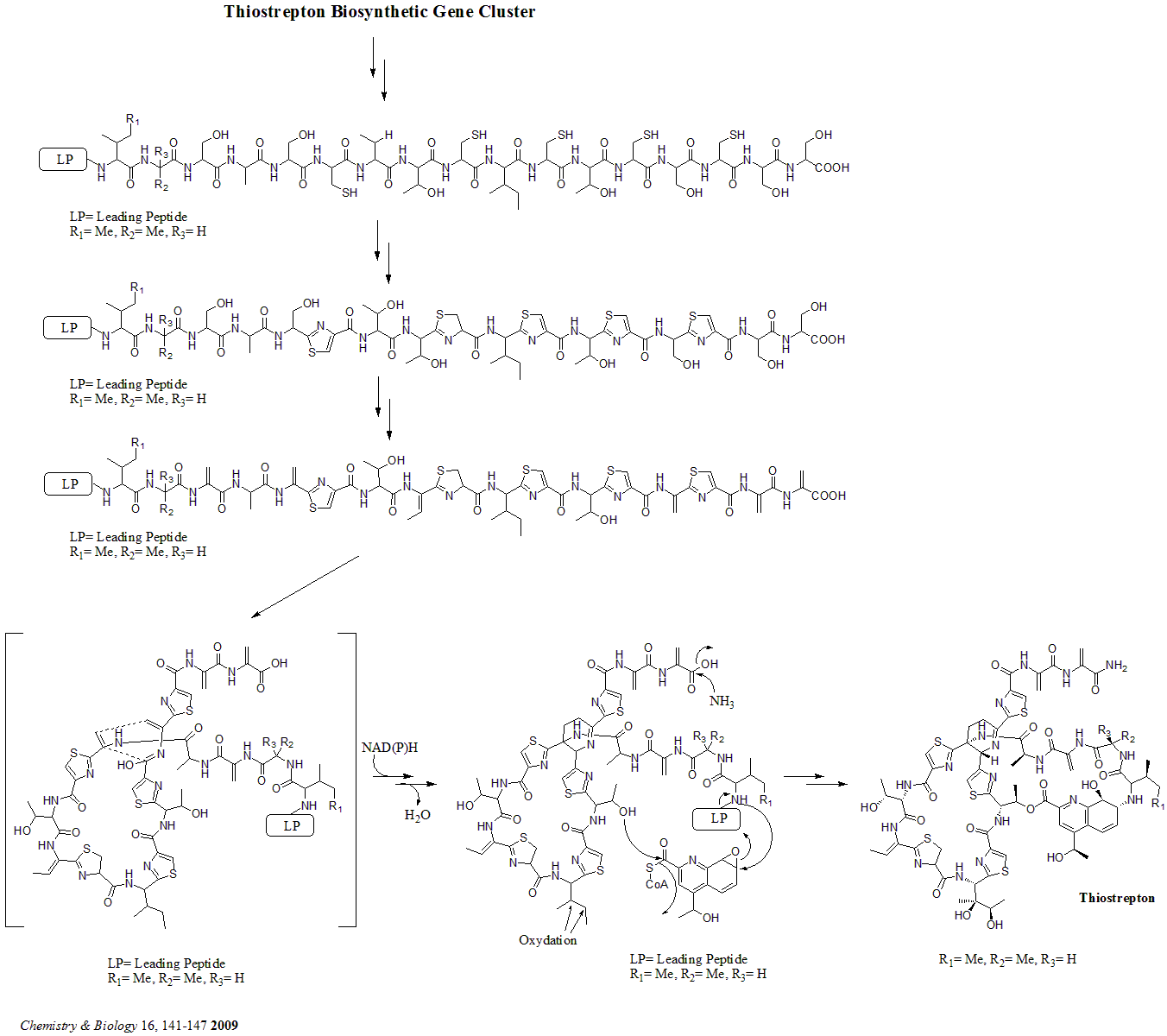
Thiostrepton
Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as ''Streptomyces azureus'' and ''Streptomyces laurentii''. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class. History Thiostrepton was discovered by Donovick ''et al.'' who described its antibacterial properties in 1955. Dorothy Crowfoot Hodgkin solved the structure of thiostrepton in 1970. Early in 1978, Bycroft and Gowland proposed the biosynthesis of thiostrepton, which was still unclear until 2009. Several studies of thiopeptide biosynthesis have been contemporarily published in 2009 and two of them (Liao ''et al.'' and Kelly ''et al.'') included the similar biosynthesis of thiostrepton: it's ribosomally synthesized from thiostrepton biosynthetic genes (tsr genes) and posttranslational modification is needed. A total synthesis of thiostrepton was completed by K.C. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)