|
Thiopeptide
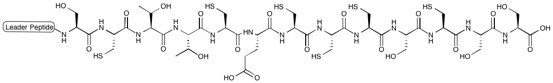
Thiopeptides (thiazolyl peptides) are a class of peptide antibiotics produced by bacteria. They have antibiotic activity against Gram-positive bacteria, but little or no activity against Gram-negative bacteria. Many of the members of this class show activity against methicillin-resistant ''Staphylococcus aureus'' (MRSA) and are therefore subjects of research interest. There are over 100 members of this class known. Chemical structure Thiopeptides are sulfur-rich macrocyclic peptides containing highly-modified amino acids. They are characterized by a nitrogen-containing six-membered ring (such as piperidine, dehydropiperidine, or pyridine) substituted with multiple thiazole rings and dehydroamino acids. A macrocylic ring serves as a scaffold for a tail that also incorporates modified amino acids often with azole rings, such as thiazoles, oxazoles, and thiazolines which are derived from serine, threonine, and cysteine residues. Examples Examples of thiopeptides include thiost ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactocillin
Lactocillin is a thiopeptide antibiotic which is encoded for and produced by biosynthetic genes clusters in the bacteria ''Lactobacillus gasseri''. Lactocillin was discovered and purified in 2014. ''Lactobacillus gasseri'' is one of the four ''Lactobacillus'' bacteria found to be most common in the human vaginal microbiome. Due to increasing levels of pathogenic resistance to known antibiotics, novel antibiotics are increasingly valuable. Lactocillin could function as a new antibiotic that could help people fight off infections that are resistant to many other antibiotics. Biosynthetic Gene Clusters Lactocillin is produced by a biosynthetic gene cluster, which is a group of genes in bacteria that work together to make a secondary metabolite. Secondary metabolites are molecules with many different chemical structures and functions, and in this case, lactocillin functions as an antibiotic. Biosynthetic gene clusters are similar to operons in bacteria in that they both code for p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nosiheptide
Nosiheptide is a thiopeptide antibiotic produced by the bacterium '' Streptomyces actuosus''. Chemical classification Nosiheptide is classified, along with several others, as an e series thiopeptide characterized by a nitrogen containing, 6-membered heterocycle in a 2,3,5,6 substituted fashion central to multiple azoles (or azolines) and dehydroamino acids along with a macrocyclic core. Nosiheptide is constructed solely of proteinogenic amino acids and has ribosomal origin, making it a member of the ribosomally synthesized and posttranslationally modified peptide family of natural products. Thiopeptides such as nosiheptide have potent activity against various bacterial pathogens, primarily gram positive, including methicillin-resistant Staphylococcus aureus, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci. Composition Nosiheptide consists of 5 thiazole rings, a central tetrasubstituted pyridine moiety, and a bicyclic macrocycle, which incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
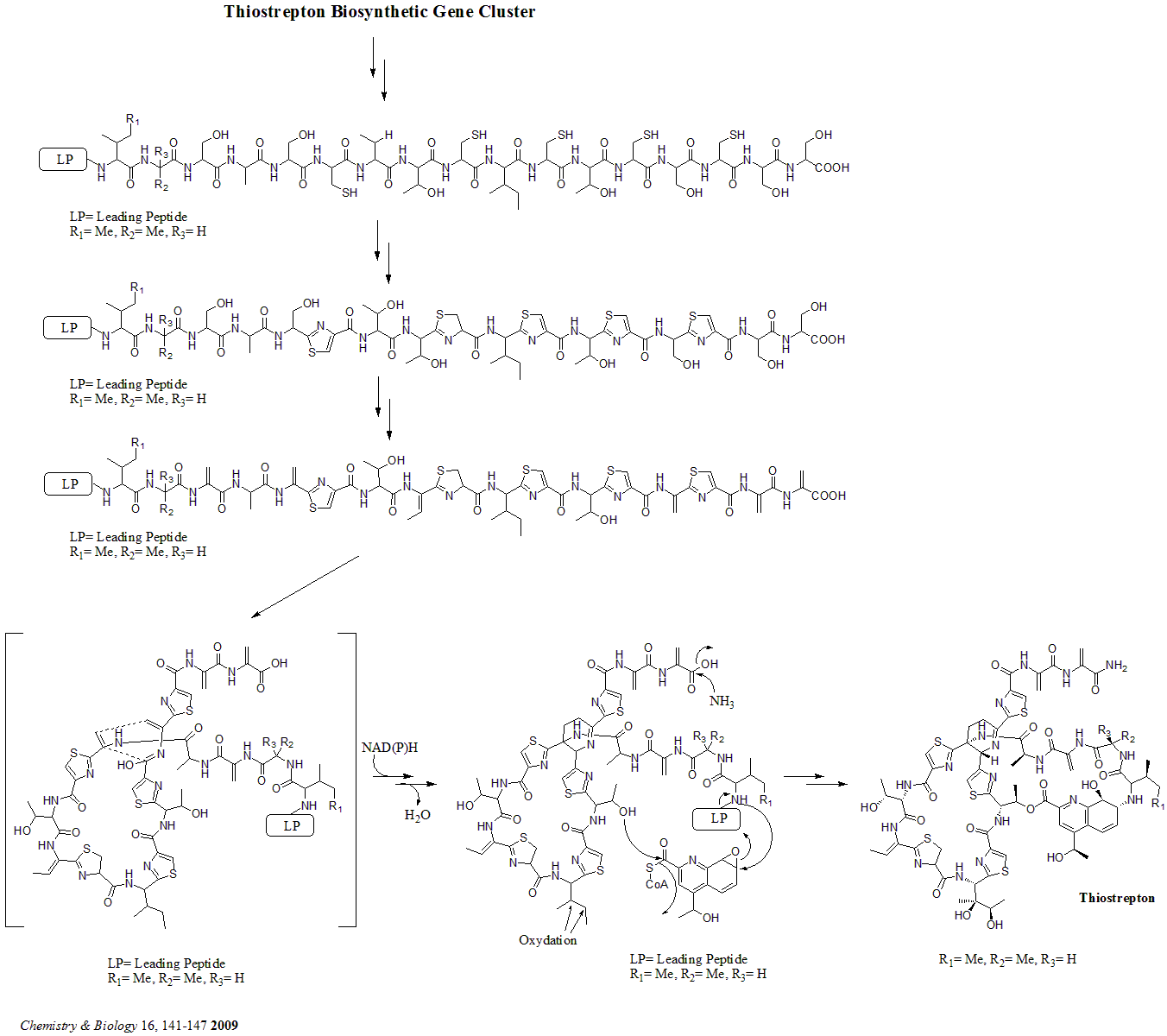
Thiostrepton
Thiostrepton is a natural cyclic oligopeptide antibiotic of the thiopeptide class, derived from several strains of streptomycetes, such as '' Streptomyces azureus'' and '' Streptomyces laurentii''. Thiostrepton is a natural product of the ribosomally synthesized and post-translationally modified peptide (RiPP) class. History Thiostrepton was discovered by Donovick ''et al.'' who described its antibacterial properties in 1955. Dorothy Crowfoot Hodgkin solved the structure of thiostrepton in 1970. Early in 1978, Bycroft and Gowland proposed the biosynthesis of thiostrepton, which was still unclear until 2009. Several studies of thiopeptide biosynthesis have been contemporarily published in 2009 and two of them (Liao ''et al.'' and Kelly ''et al.'') included the similar biosynthesis of thiostrepton: it's ribosomally synthesized from thiostrepton biosynthetic genes (tsr genes) and posttranslational modification is needed. A total synthesis of thiostrepton was completed by K.C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclothiazomycin
The cyclothiazomycins are a group of natural products, classified as thiopeptides, which are produced by various ''Streptomyces'' species of bacteria. These compounds are ribosomally synthesized and post-translationally modified peptides (RiPPs) and can be further classified as thiopeptides. The overall structure of the cyclothiazomycins comprises a macrocyclic bicyclic peptide containing several thiazoles and thiazolines. The cyclothiazomycins are reported to have multiple inhibitory effects ranging from decreasing blood pressure to interfering with RNA transcription; they also exhibit some antibiotic activity. History Cylothiazomycin A was first isolated from ''Streptomyces'' sp. NR0516 in 1991. The structure of cyclothiazomycin A was solved via NMR spectroscopy and chemical degradation. Previously, a peptide compound 5102-II had been isolated in 1982 from ''Streptomyces hygroscopicus'' 10-22. The discovery of the genes responsible for the biosynthesis of cyclothiazomy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorganism fighting another), whereas non-antibiotic antibacterials (such as sulfonamides and antise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-positive Bacteria
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall. Gram-positive bacteria take up the crystal violet stain used in the test, and then appear to be purple-coloured when seen through an optical microscope. This is because the thick peptidoglycan layer in the bacterial cell wall retains the stain after it is washed away from the rest of the sample, in the decolorization stage of the test. Conversely, gram-negative bacteria cannot retain the violet stain after the decolorization step; alcohol used in this stage degrades the outer membrane of gram-negative cells, making the cell wall more porous and incapable of retaining the crystal violet stain. Their peptidoglycan layer is much thinner and sandwiched between an inner cell membrane and a bacterial outer membrane, causing them to take up the counterstain ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram-negative Bacteria
Gram-negative bacteria are bacteria that do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. They are characterized by their cell envelopes, which are composed of a thin peptidoglycan cell wall sandwiched between an inner cytoplasmic cell membrane and a bacterial outer membrane. Gram-negative bacteria are found in virtually all environments on Earth that support life. The gram-negative bacteria include the model organism '' Escherichia coli'', as well as many pathogenic bacteria, such as '' Pseudomonas aeruginosa'', '' Chlamydia trachomatis'', and '' Yersinia pestis''. They are a significant medical challenge as their outer membrane protects them from many antibiotics (including penicillin), detergents that would normally damage the inner cell membrane, and lysozyme, an antimicrobial enzyme produced by animals that forms part of the innate immune system. Additionally, the outer leaflet of this membrane comprises a compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methicillin-resistant Staphylococcus Aureus
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths attributable to antimicrobial resistance in 2019. MRSA is any strain of ''S. aureus'' that has developed (through natural selection) or acquired (through horizontal gene transfer) a multiple drug resistance to beta-lactam antibiotics. Beta-lactam (β-lactam) antibiotics are a broad-spectrum group that include some penams (penicillin derivatives such as methicillin and oxacillin) and cephems such as the cephalosporins. Strains unable to resist these antibiotics are classified as methicillin-susceptible ''S. aureus'', or MSSA. MRSA is common in hospitals, prisons, and nursing homes, where people with open wounds, invasive devices such as catheters, and weakened immune systems are at greater risk of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrocyclic
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry. Synthesis The formation of macrocycles by ring-closure is called macrocylization. Pioneering work was reported for studies on terpenoid macrocycles. The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, small rings or polymers tend to form. This kinetic problem can be addressed by using high-dilution reactions, whereby intramolecular processes are favored relative to polymerizations. Some macrocyclizations are favored using template reactions. Templates are ions, molecules, surfaces etc. that bind and pre-organize compounds, guiding them toward formation of a particular ring size. The crown ethers are often generated in the presence of an alkali metal cation, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group. Oxazoles are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazoles, with the thiazole sulfur replaced by nitrogen. Thiazole rings are planar and aromatic. Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazoles and have therefore greater aromaticity. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR spectroscopy (between 7.27 and 8.77 ppm), clearly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |