Nosiheptide on:
[Wikipedia]
[Google]
[Amazon]
Nosiheptide is a
 All moieties of the peptidyl backbone of nosiheptide were shown to originate exclusively from proteinogenic amino acids. The structural motifs include dehydroamino acids from the
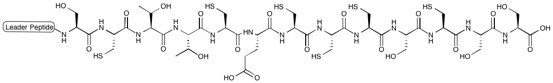
All moieties of the peptidyl backbone of nosiheptide were shown to originate exclusively from proteinogenic amino acids. The structural motifs include dehydroamino acids from the 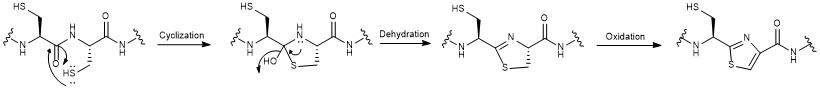 Nosiheptide biosynthesis begins with the translation of a 50 amino acid precursor peptide consisting of a 37 amino acid leader peptide fused to a 13 amino acid structural peptide (SCTTCECCCSCSS), matching the nosiheptide backbone aside from the C-terminal serine residue that is partially cleaved in maturation of the final product. Next, cyclizations occur between 5 of the cysteine residues in the structural sequence and the previous carbonyl on the adjacent amino acid to form thiazole rings after oxidation. Several amino acid residues then undergo elimination of water to produce dehydroamino acids, and the first macrocycle is constructed by cyclization of two of these dehydroamino acid residues. This cyclization includes a Diels-Alder type reaction, dehydration, and elimination of the leader peptide. An indolic acid moiety modified from L-tryptophan is then ligated to the unmodified cysteine residue through a
Nosiheptide biosynthesis begins with the translation of a 50 amino acid precursor peptide consisting of a 37 amino acid leader peptide fused to a 13 amino acid structural peptide (SCTTCECCCSCSS), matching the nosiheptide backbone aside from the C-terminal serine residue that is partially cleaved in maturation of the final product. Next, cyclizations occur between 5 of the cysteine residues in the structural sequence and the previous carbonyl on the adjacent amino acid to form thiazole rings after oxidation. Several amino acid residues then undergo elimination of water to produce dehydroamino acids, and the first macrocycle is constructed by cyclization of two of these dehydroamino acid residues. This cyclization includes a Diels-Alder type reaction, dehydration, and elimination of the leader peptide. An indolic acid moiety modified from L-tryptophan is then ligated to the unmodified cysteine residue through a 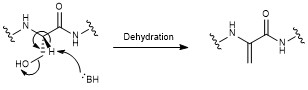
 The
The
thiopeptide
Thiopeptides (thiazolyl peptides) are a class of peptide antibiotics produced by bacteria. They have antibiotic activity against Gram-positive bacteria, but little or no activity against Gram-negative bacteria. Many of the members of this class s ...
antibiotic produced by the bacterium ''Streptomyces actuosus
''Streptomyces actuosus'' is a bacterium species from the genus of '' Streptomyces''. ''Streptomyces actuosus'' produces nosiheptide
Nosiheptide is a thiopeptide antibiotic produced by the bacterium '' Streptomyces actuosus''.
Chemical clas ...
''.
Chemical classification
Nosiheptide is classified, along with several others, as an e series thiopeptide characterized by a nitrogen containing, 6-memberedheterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis ...
in a 2,3,5,6 substituted fashion central to multiple azole
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxygen) as part of the ring.
Their names originate from the Hantzsch–Widman nomenclature. Th ...
s (or azolines) and dehydroamino acids along with a macrocyclic core. Nosiheptide is constructed solely of proteinogenic amino acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino aci ...
s and has ribosomal
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to fo ...
origin, making it a member of the ribosomally synthesized and posttranslationally modified peptide family of natural products. Thiopeptides such as nosiheptide have potent activity against various bacterial pathogen
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
s, primarily gram positive
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
Gram-positive bact ...
, including methicillin-resistant Staphylococcus aureus
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of Gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. ...
, penicillin-resistant Streptococcus pneumoniae, and vancomycin-resistant enterococci.
Composition
Nosiheptide consists of 5thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular for ...
rings, a central tetrasubstituted pyridine moiety, and a bicyclic macrocycle
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
, which includes a modified amino acid (from tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
) external to the initial peptide translated from the gene encoding it. It is used as a feed additive in the growth of poultry and hogs to promote growth and general health, although it has not been applied in human medicines due to low water solubility and poor resorption from the gastrointestinal tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organ (biology), organs of the digestive syste ...
. Nosiheptide and other thiopeptide's mechanism of action
In pharmacology, the term mechanism of action (MOA) refers to the specific biochemical interaction through which a drug substance produces its pharmacological effect. A mechanism of action usually includes mention of the specific molecular targe ...
stems from the tight binding on the 50S ribosomal subunit and inhibiting the activities of elongation factors, preventing protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
synthesis.
Biosynthesis
 All moieties of the peptidyl backbone of nosiheptide were shown to originate exclusively from proteinogenic amino acids. The structural motifs include dehydroamino acids from the
All moieties of the peptidyl backbone of nosiheptide were shown to originate exclusively from proteinogenic amino acids. The structural motifs include dehydroamino acids from the serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
or threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO� ...
residues undergoing anti elimination of water, thiazoles from cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, sometime ...
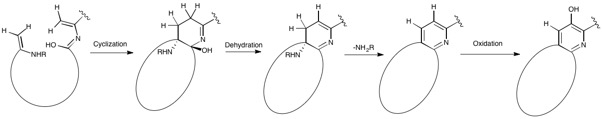
residues with cyclodehydration followed by deoxygenation, and the central hydroxypyridine produced by cyclization between two dehydroalanine acids with incorporation of an adjacent carbonyl group.
 Nosiheptide biosynthesis begins with the translation of a 50 amino acid precursor peptide consisting of a 37 amino acid leader peptide fused to a 13 amino acid structural peptide (SCTTCECCCSCSS), matching the nosiheptide backbone aside from the C-terminal serine residue that is partially cleaved in maturation of the final product. Next, cyclizations occur between 5 of the cysteine residues in the structural sequence and the previous carbonyl on the adjacent amino acid to form thiazole rings after oxidation. Several amino acid residues then undergo elimination of water to produce dehydroamino acids, and the first macrocycle is constructed by cyclization of two of these dehydroamino acid residues. This cyclization includes a Diels-Alder type reaction, dehydration, and elimination of the leader peptide. An indolic acid moiety modified from L-tryptophan is then ligated to the unmodified cysteine residue through a
Nosiheptide biosynthesis begins with the translation of a 50 amino acid precursor peptide consisting of a 37 amino acid leader peptide fused to a 13 amino acid structural peptide (SCTTCECCCSCSS), matching the nosiheptide backbone aside from the C-terminal serine residue that is partially cleaved in maturation of the final product. Next, cyclizations occur between 5 of the cysteine residues in the structural sequence and the previous carbonyl on the adjacent amino acid to form thiazole rings after oxidation. Several amino acid residues then undergo elimination of water to produce dehydroamino acids, and the first macrocycle is constructed by cyclization of two of these dehydroamino acid residues. This cyclization includes a Diels-Alder type reaction, dehydration, and elimination of the leader peptide. An indolic acid moiety modified from L-tryptophan is then ligated to the unmodified cysteine residue through a thioester
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by t ...
linkage. After methylation and oxidation of the indole, the second macrocycle is formed by reaction of the indole hydroxyl group with the free carboxyl group of the nearby glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
residue. The now fused bicyclic peptide undergoes several oxidations and partial cleavage of the C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
dehydroalanine residue to give the mature nosiheptide.

 The
The translation
Translation is the communication of the Meaning (linguistic), meaning of a #Source and target languages, source-language text by means of an Dynamic and formal equivalence, equivalent #Source and target languages, target-language text. The ...
and modification of the precursor peptide is undertaken by several enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s encoded in a localized gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a ba ...
cluster containing 14 structural genes and 1 regulatory gene. One of these genes encodes the peptide, and the others are responsible for the posttranslational modifications.
Total synthesis
The first total synthesis was published by Wojtas ''et al.'' in 2016. It was achieved through the assembly of a fully functionalized linear precursor followed by consecutive macrocyclizations. Key features are a critical macrothiolactonization and a mild deprotection strategy for the 3-hydroxypyridine core. The natural product was identical to isolated authentic material in terms of spectral data and antibiotic activity.References
{{reflist, refs= {{cite journal , last1 = Yu , display-authors=etal , year = 2009 , title = Nosiheptide Biosynthesis Featuring a Unique Indole Side Ring Formation on the Characteristic Thiopeptide Framework , journal = ACS Chemical Biology , volume = 4 , issue = 10, pages = 855–864 , doi=10.1021/cb900133x, pmc = 2763056 , pmid=19678698 {{cite journal , last1 = Bagley , display-authors=etal , year = 2005 , title = Thiopeptide Antibiotics , journal = Chem. Rev. , volume = 105 , issue = 2, pages = 685–714 , doi=10.1021/cr0300441 , pmid=15700961 {{cite journal , last1 = Benazet , first1 = F. , display-authors=etal , year = 1980 , title = Nosiheptide, a sulfur-containing peptide antibiotic isolated from Streptomyces actuosus 40037 , journal = Experientia , volume = 36 , issue = 4, pages = 414–416 , doi=10.1007/bf01975121 , pmid=7379912 {{cite journal , last1 = Mocek , display-authors=etal , year = 1993 , title = Biosynthesis of the Modified Peptide Antibiotic Nosiheptide in Streptomyces actuosus , journal = Journal of the American Chemical Society , volume = 115 , issue = 17, pages = 7558–7568 , doi=10.1021/ja00070a001 {{cite journal , last1 = Cundliffe , first1 = E. , last2 = Thompson , first2 = J. , year = 1981 , title = The Mode of Action of Nosiheptide (Multhiomycin) and the Mechanism of Resistance in the Producing Organism, doi = 10.1099/00221287-126-1-185 , pmid = 7038038 , journal = J. Gen. Microbiol. , volume = 126 , issue = 1, pages = 185–192 , doi-access = free {{cite journal , last1 = Wang , first1 = S. , last2 = Zhou , first2 = S. , last3 = Liu , first3 = W. , year = 2013 , title = Opportunities and challenges from current investigations into the biosynthetic logic of nosiheptide-represented thiopeptide antibiotics, journal = Current Opinion in Chemical Biology , volume = 17 , issue = 4, pages = 626–634 , doi=10.1016/j.cbpa.2013.06.021, pmid = 23838388 {{cite journal , last1 = Guo , display-authors=etal , year = 2014 , title = Insight into bicyclic thiopeptide biosynthesis benefited from development of a uniform approach for molecular engineering and production improvement, journal = Chemical Science , volume = 5 , pages = 240–246 , doi=10.1039/c3sc52015c {{cite journal , last1 = Yu , display-authors=etal , year = 2010 , title = NosA Catalyzing Carboxyl-Terminal Amide Formation in Nosiheptide Maturation via an Enamine Dealkylation on the Serine-Extended Precursor Peptide , journal = Journal of the American Chemical Society , volume = 132 , issue = 46, pages = 16324–16326 , doi=10.1021/ja106571g, pmc = 2990472 , pmid=21047073 Paul M. Dewick, ''Medicinal Natural Products: A Biosynthetic Approach, 3rd Ed''., 2009, John Wiley & Sons, pg. 86 & 443 Thiopeptides