|
Bottromycin
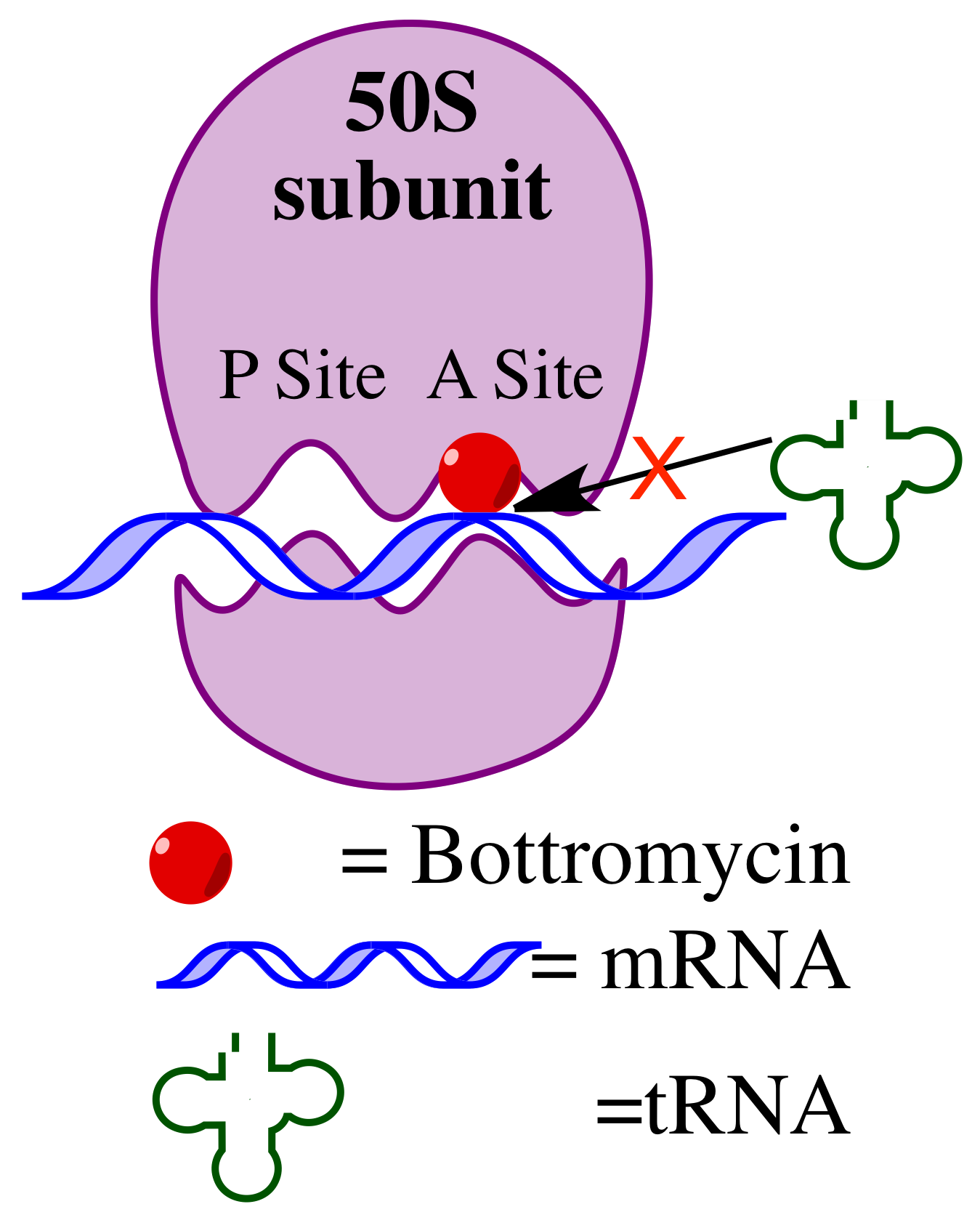
Bottromycin is a macrocyclic peptide with antibiotic activity. It was first discovered in 1957 as a natural product isolated from '' Streptomyces bottropensis''. It has been shown to inhibit methicillin-resistant ''Staphylococcus aureus'' (MRSA) and vancomycin-resistant Enterococci ( VRE) among other Gram-positive bacteria and mycoplasma. Bottromycin is structurally distinct from both vancomycin, a glycopeptide antibiotic, and methicillin, a beta-lactam antibiotic. Bottromycin binds to the A site of the ribosome and blocks the binding of aminoacyl-''t''RNA, therefore inhibiting bacterial protein synthesis. Although bottromycin exhibits antibacterial activity ''in vitro'', it has not yet been developed as a clinical antibiotic, potentially due to its poor stability in blood plasma. To increase its stability ''in vivo'', some bottromycin derivatives have been explored. The structure of bottromycin contains a macrocyclic amidine as well as a thiazole ring. The absolute stereochemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RiPPs
Ribosomally synthesized and post-translationally modified peptides (RiPPs), also known as ribosomal natural products, are a diverse class of natural products of ribosomal origin. Consisting of more than 20 sub-classes, RiPPs are produced by a variety of organisms, including prokaryotes, eukaryotes, and archaea, and they possess a wide range of biological functions. As a consequence of the falling cost of genome sequencing and the accompanying rise in available genomic data, scientific interest in RiPPs has increased in the last few decades. Because the chemical structures of RiPPs are more closely predictable from genomic data than are other natural products (e.g. alkaloids, terpenoids), their presence in sequenced organisms can, in theory, be identified rapidly. This makes RiPPs an attractive target of modern natural product discovery efforts. Definition RiPPs consist of any peptides (i.e. molecular weight below 10 kDa) that are ribosomally-produced and undergo some degree ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptomyces
''Streptomyces'' is the largest genus of Actinomycetota and the type genus of the family Streptomycetaceae. Over 500 species of ''Streptomyces'' bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Streptomycetes are characterised by a complex secondary metabolism. They produce over two-thirds of the clinically useful antibiotics of natural origin (e.g., neomycin, streptomycin, cypemycin, grisemycin, bottromycins and chloramphenicol). The antibiotic streptomycin takes its name directly from ''Streptomyces''. Streptomycetes are infrequent pathogens, though infections in humans, such as mycetoma, can be caused by '' S. somaliensis'' and '' S. sudanensis'', and in plants can be caused by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptomyces Bottropensis
''Streptomyces bottropensis'' is a bacterium species from the genus ''Streptomyces'' which has been isolated from soil.Deutsche Sammlung von Mikroorganismen und Zellkulturenbr>/ref> ''Streptomyces bottropensis'' produces bottromycin, dunaimycin and mensacarcin. ''Streptomyces bottropensis'' can metabolize (+)-carvone Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill. Uses Both carv ... to (+)- bottrospicatol. See also * List of ''Streptomyces'' species References Further reading * * * * * * * * * External linksType strain of ''Streptomyces bottropensis'' at Bac''Dive'' - the Bacterial Diversity Metadatabase bottropensis Bacteria described in 1961 {{Streptomyces-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transcriptional Regulator
In molecular biology and genetics, transcriptional regulation is the means by which a cell regulates the conversion of DNA to RNA ( transcription), thereby orchestrating gene activity. A single gene can be regulated in a range of ways, from altering the number of copies of RNA that are transcribed, to the temporal control of when the gene is transcribed. This control allows the cell or organism to respond to a variety of intra- and extracellular signals and thus mount a response. Some examples of this include producing the mRNA that encode enzymes to adapt to a change in a food source, producing the gene products involved in cell cycle specific activities, and producing the gene products responsible for cellular differentiation in multicellular eukaryotes, as studied in evolutionary developmental biology. The regulation of transcription is a vital process in all living organisms. It is orchestrated by transcription factors and other proteins working in concert to finely tune t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonribosomal Peptide
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term ''nonribosomal peptide'' typically refers to a very specific set of these as discussed in this article. Nonribosomal peptides are synthesized by nonribosomal peptide synthetases, which, unlike the ribosomes, are independent of messenger RNA. Each nonribosomal peptide synthetase can synthesize only one type of peptide. Nonribosomal peptides often have cyclic and/or branched structures, can contain non- proteinogenic amino acids including D-amino acids, carry modifications like '' N''-methyl and ''N''-formyl groups, or are glycosylated, acylated, halogenated, or hydroxylated. Cyclization of amino acids against the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the system. In the most common version, the trajectories of atoms and molecules are determined by numerically solving Newton's equations of motion for a system of interacting particles, where forces between the particles and their potential energies are often calculated using interatomic potentials or molecular mechanical force fields. The method is applied mostly in chemical physics, materials science, and biophysics. Because molecular systems typically consist of a vast number of particles, it is impossible to determine the properties of such complex systems analytically; MD simulation circumvents this problem by using numerical methods. However, long MD simulations are mathematically ill-conditioned, generating cumulative err ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4− c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paper Chromatography
Paper chromatography is an analytical method used to separate coloured chemicals or substances. It is now primarily used as a teaching tool, having been replaced in the laboratory by other chromatography methods such as thin-layer chromatography (TLC). A paper chromatography variant, two-dimensional chromatography, involves using two solvents and rotating the paper 90° in between. This is useful for separating complex mixtures of compounds having similar polarity, for example, amino acids. The setup has three components. The mobile phase is a solution that travels up the stationary phase, due to capillary action. The mobile phase is generally a mixture of non-polar organic solvent, while the stationary phase is polar inorganic solvent water. Here paper is used to support the stationary phase, water. Polar water molecules are held inside the void space of the cellulose network of the host paper. The difference between TLC and paper chromatography is that the stationary phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ninhydrin
Ninhydrin (2,2-dihydroxyindane-1,3-dione) is an organic compound with the formula C6H4(CO)2C(OH)2. It is used to detect ammonia and amines. Upon reaction with these amines, ninhydrin gets converted into deep blue or purple derivatives, which are called Ruhemann's purple. Ninhydrin is most commonly used to detect fingerprints, as the terminal amines of lysine residues in peptides and proteins sloughed off in fingerprints react with ninhydrin. Ninhydrin is a white solid that is soluble in ethanol and acetone. Ninhydrin can be considered as the hydrate of indane-1,2,3-trione. History Ninhydrin was discovered in 1910 by the German-English chemist Siegfried Ruhemann (1859–1943). In the same year, Ruhemann observed ninhydrin's reaction with amino acids. In 1954, Swedish investigators Oden and von Hofsten proposed that ninhydrin could be used to develop latent fingerprints. Uses Ninhydrin can also be used to monitor deprotection in solid phase peptide synthesis (Kaiser test). The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylation
: In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Organic synthesis Acetate esters and acetamides are generally prepared by acetylations. Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions. Carbanions and their equivalents are susceptible to acetylations. Acetylation reagents Many acetylations are achieved using these three reagents: *Acetic anhydride. This reagent is common in the laboratory; its use cogenerates acetic acid. * Acetyl chloride. This reagent is also common in the laboratory, but its use cogenerates hydrogen chloride, which can be undesirable. * Ketene. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: :H2C=C=O + CH3COOH -> (CH3C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |