|
Acetylation
: In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opposite reaction, the removal of an acetyl group from a chemical compound. Organic synthesis Acetate esters and acetamides are generally prepared by acetylations. Acetylations are often used in making C-acetyl bonds in Friedel-Crafts reactions. Carbanions and their equivalents are susceptible to acetylations. Acetylation reagents Many acetylations are achieved using these three reagents: *Acetic anhydride. This reagent is common in the laboratory; its use cogenerates acetic acid. * Acetyl chloride. This reagent is also common in the laboratory, but its use cogenerates hydrogen chloride, which can be undesirable. * Ketene. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: :H2C=C=O + CH3COOH -> (CH3C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Post-translational Modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones. Post-translational modifications can occur on the amino acid side chains or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acids by modifying an existing functional group or introducing a new one such as phosphate. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic and prokaryotic proteins also have carbohydrate molecules attached to them in a process calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histone Acetyltransferase
Histone acetyltransferases (HATs) are enzymes that acetylate conserved lysine amino acids on histone proteins by transferring an acetyl group from acetyl-CoA to form ε-''N''-acetyllysine. DNA is wrapped around histones, and, by transferring an acetyl group to the histones, genes can be turned on and off. In general, histone acetylation increases gene expression. In general, histone acetylation is linked to transcriptional activation and associated with euchromatin. Euchromatin, which is less densely compact, allows transcription factors to bind more easily to regulatory sites on DNA, causing transcriptional activation. When it was first discovered, it was thought that acetylation of lysine neutralizes the positive charge normally present, thus reducing affinity between histone and (negatively charged) DNA, which renders DNA more accessible to transcription factors. Research has emerged, since, to show that lysine acetylation and other posttranslational modifications of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl Group
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, although this term is barely heard. The acetyl group contains a methyl group () single-bonded to a carbonyl (). The carbonyl center of an acyl radical has one nonbonded electron with which it forms a chemical bond to the remainder ''R'' of the molecule. The acetyl moiety is a component of many organic compounds, including acetic acid, the neurotransmitter acetylcholine, acetyl-CoA, acetylcysteine, acetaminophen (also known as paracetamol), and acetylsalicylic acid (also known as aspirin). Acetylation In nature The introduction of an acetyl group into a molecule is called acetylation. In biological organisms, acetyl groups are commonly transferred from acetyl-CoA to other organic molecules. Acetyl-CoA is an intermediate both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air. Structure and properties Acetic anhydride, like most acid anhydrides, is a flexible molecule with a nonplanar structure. The pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low. Like most acid anhydrides, the carbonyl carbon atom of acetic anhydride has electrophilic character, as the leaving group is carboxylate. The internal asymmetry may contribute to acetic anhydride's potent electrophilicity as the asymmetric geomet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspirin Synthesis
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease, pericarditis, and rheumatic fever. Aspirin is also used long-term to help prevent further heart attacks, ischaemic strokes, and blood clots in people at high risk. For pain or fever, effects typically begin within 30 minutes. Aspirin works similarly to other NSAIDs but also suppresses the normal functioning of platelets. One common adverse effect is an upset stomach. More significant side effects include stomach ulcers, stomach bleeding, and worsening asthma. Bleeding risk is greater among those who are older, drink alcohol, take other NSAIDs, or are on other blood thinners. Aspirin is not recommended in the last part of pregnancy. It is not generally recommended in children with infections because of the risk of Re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histone Deacetylase
Histone deacetylases (, HDAC) are a class of enzymes that remove acetyl groups (O=C-CH3) from an ε-N-acetyl lysine amino acid on a histone, allowing the histones to wrap the DNA more tightly. This is important because DNA is wrapped around histones, and DNA expression is regulated by acetylation and de-acetylation. Its action is opposite to that of histone acetyltransferase. HDAC proteins are now also called lysine deacetylases (KDAC), to describe their function rather than their target, which also includes non-histone proteins. HDAC super family Together with the acetylpolyamine amidohydrolases and the acetoin utilization proteins, the histone deacetylases form an ancient protein superfamily known as the histone deacetylase superfamily. Classes of HDACs in higher eukaryotes HDACs, are classified in four classes depending on sequence homology to the yeast original enzymes and domain organization: HDAC (except class III) contain zinc and are known as Zn2+-dependen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
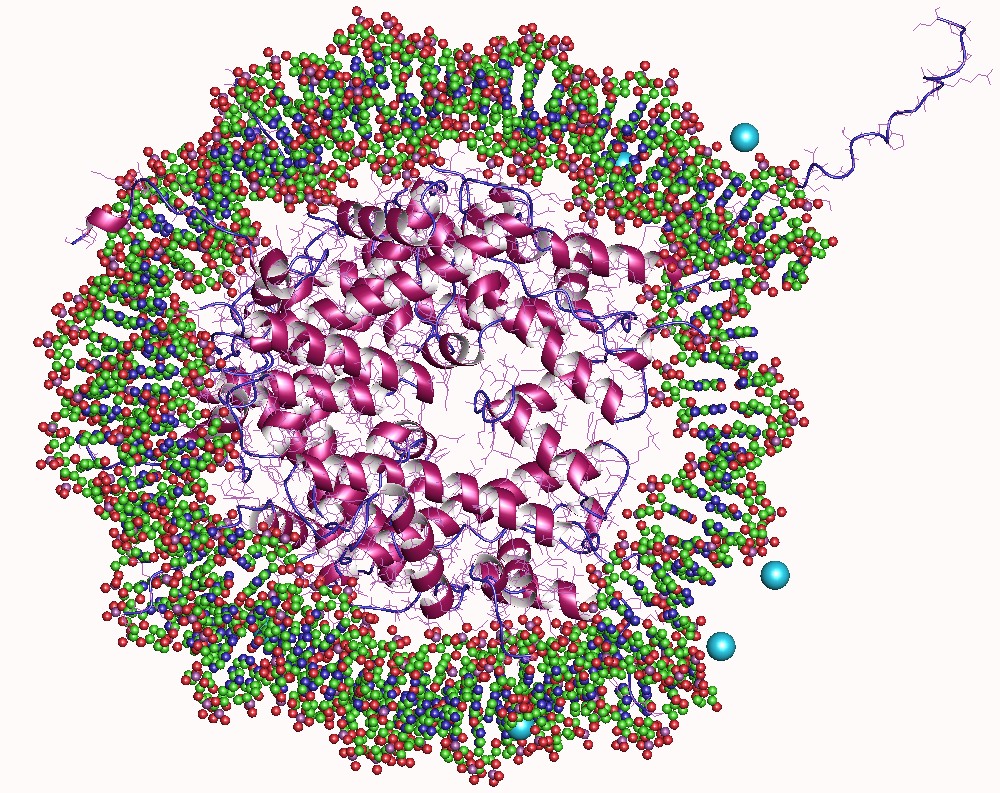
Histone Acetylation And Deacetylation
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30-nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 mm) of 30 nm diameter chromatin fibers. There are five families of histones which are designated H1/H5 (linker histones), H2, H3, and H4 (core histones). The nucleosome core is formed of two H2A-H2B dimers and a H3-H4 tetramer. The tight wrapping of DNA around hist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetoxy Group
In organic chemistry, the acetoxy group (abbr. AcO or OAc; IUPAC name: acetyloxy), is a functional group with the formula and the structure . As the ''-oxy'' suffix implies, it differs from the acetyl group () by the presence of an additional oxygen atom. The name acetoxy is the short form of ''acetyl-oxy''. Functionality An acetoxy group may be used as a protection for an alcohol functionality in a synthetic route although the protecting group itself is called an acetyl group. Alcohol protection There are several options of introducing an acetoxy functionality in a molecule from an alcohol (in effect protecting the alcohol by acetylation): * Acetyl halide, such as acetyl chloride in the presence of a base like triethylamine * Activated ester form of acetic acid, such as a N-hydroxysuccinimide ester, although this is not advisable due to higher costs and difficulties. * Acetic anhydride in the presence of base with a catalyst such as pyridine with a bit of DMAP added ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common building block for biosynthesis. Nomenclature and common formula When part of a salt, the formula of the acetate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |