benzylic on:
[Wikipedia]
[Google]
[Amazon]
 In
In
CH3C6H4CH3 + 3 O2 -> HO2CC6H4CO2H + 2 H2O
Millions of tonnes of terephthalic acid are produced annually by this method.
 * Monobenzylation of
* Monobenzylation of
 * Single electron process with Na/ NH3 or Li/NH3
* Benzyl protecting groups can be removed using a wide range of oxidizing agents including:
** CrO3/
* Single electron process with Na/ NH3 or Li/NH3
* Benzyl protecting groups can be removed using a wide range of oxidizing agents including:
** CrO3/
 * Strong base such as powdered
* Strong base such as powdered 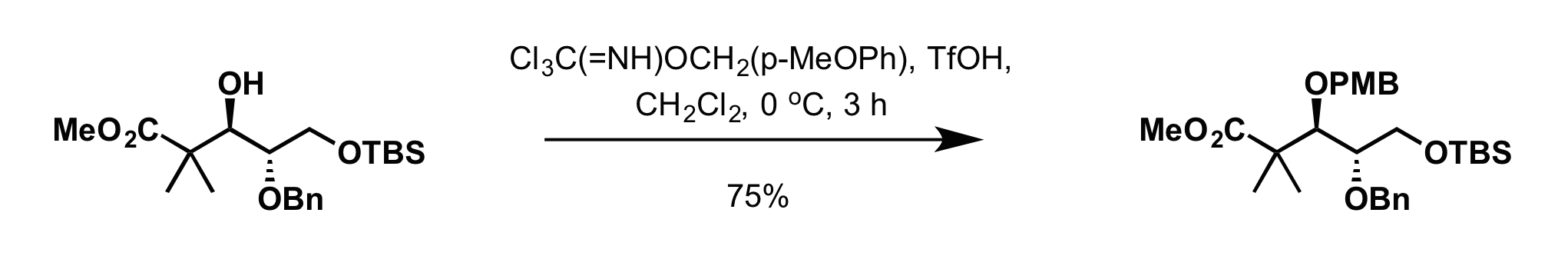
 * Conditions for deprotection of benzyl group are applicable for cleavage of the PMB protecting group
* Conditions for deprotection of benzyl group are applicable for cleavage of the PMB protecting group
 *
* 

organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, benzyl is the substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as ''sid ...
or molecular fragment possessing the structure . Benzyl features a benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
ring () attached to a methylene group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as , where the ' ...
() group.
Nomenclature
InIUPAC nomenclature
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
The ...
, the prefix benzyl refers to a substituent, for example benzyl chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
Preparation
Benzyl chloride is prepared indus ...
or benzyl benzoate
Benzyl benzoate is an organic compound which is used as a medication and insect repellent. As a medication it is used to treat scabies and lice. For scabies either permethrin or malathion is typically preferred. It is applied to the skin as a lot ...
. Benzyl is not to be confused with phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydroge ...
with the formula .
The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satu ...
ring. For example, is referred to as a "benzylic" carbocation. The benzyl free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
has the formula . The benzyl cation or phenylcarbenium ion is the carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encounte ...
with formula ; the benzyl anion or phenylmethanide ion is the carbanion with the formula . None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
s and may exist as reactive intermediates.
Abbreviations
The abbreviation "Bn" denotes benzyl. For example,benzyl alcohol
Benzyl alcohol is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid ...
can be represented as BnOH. This abbreviation is not to be confused with "Bz", which is the abbreviation for the benzoyl
In organic chemistry, benzoyl (, ) is the functional group with the formula C6H5CO-. It can be viewed as benzaldehyde missing one hydrogen.
The term "benzoyl" should not be confused with benzyl, which has the formula C6H5CH2. The benzoyl group ...
group , or the phenyl group , abbreviated "Ph". Confusingly, in old literature, "Bz" was also used for benzyl.
Reactivity of benzylic centers
The enhanced reactivity of benzylic positions is attributed to the lowbond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
for benzylic C−H bonds. Specifically, the bond is about 10–15% weaker than other kinds of C−H bonds. The neighboring aromatic ring stabilizes benzyl radicals. The data tabulated below compare benzylic C−H bond to related C−H bond strengths.
The weakness of the C−H bond reflects the stability of the benzylic radical. For related reasons, benzylic substituents exhibit enhanced reactivity, as in oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
, free radical halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of UV light. The reaction is used for the industrial synthesis of chloroform ( ...
, or hydrogenolysis. As a practical example, in the presence of suitable catalysts, ''p''-xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are su ...
oxidizes exclusively at the benzylic positions to give terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced ann ...
:
:Functionalization at the benzylic position
In a few cases, these benzylic transformations occur under conditions suitable for lab synthesis. The Wohl-Ziegler reaction will brominate a benzylic C–H bond: (). Any non-tertiary benzylic alkyl group will be oxidized to a carboxyl group by aqueous potassium permanganate () or concentrated nitric acid (): (). Finally, the complex ofchromium trioxide
Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name.
This compound is a dark-purple ...
and 3,5-dimethylpyrazole
3,5-Dimethylpyrazole is an organic compound with the formula (CH3C)2CHN2H. It is one of several isomeric derivatives of pyrazole that contain two methyl substituents. The compound is unsymmetrical but the corresponding conjugate acid (pyrazolium ...
() will selectively oxidize a benzylic methylene group to a carbonyl: (). 2-iodoxybenzoic acid
2-Iodoxybenzoic acid (IBX) is an organic compound used in organic synthesis as an oxidizing agent. This periodinane is especially suited to oxidize alcohols to aldehydes. IBX is prepared from 2-iodobenzoic acid, potassium bromate, and sulfuric ...
in DMSO performs similarly.
As a protecting group
Benzyl groups are occasionally employed as protecting groups in organic synthesis. Their installation and especially their removal require relatively harsh conditions, so benzyl is not typically preferred for protection.Alcohol protection
Benzyl is commonly used in organic synthesis as a robust protecting group foralcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s and carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s.
* Treatment of alcohol with a strong base such as powdered potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploi ...
or sodium hydride and benzyl halide ( BnCl or BnBr)
*: * Monobenzylation of
* Monobenzylation of diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
The most common industrial diol is e ...
s can be achieved using Ag2O in dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the major ...
(DMF) at ambient to elevated temperatures
* Primary alcohol
A primary alcohol is an alcohol in which the hydroxy group is bonded to a primary carbon atom. It can also be defined as a molecule containing a “–CH2OH” group.
In contrast, a secondary alcohol has a formula “–CHROH” and a tertiary ...
s can be selectively benzylated in presence of phenol functional groups using Cu(acac)2
Deprotection methods
Benzyl ethers can be removed under '' reductive conditions'', ''oxidative
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
conditions'', and the use of ''Lewis Acids''.
* Removed using hydrogenolysis
*: * Single electron process with Na/ NH3 or Li/NH3
* Benzyl protecting groups can be removed using a wide range of oxidizing agents including:
** CrO3/
* Single electron process with Na/ NH3 or Li/NH3
* Benzyl protecting groups can be removed using a wide range of oxidizing agents including:
** CrO3/acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component ...
at ambient temperature
** Ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lowe ...
** ''N''-Bromosuccinimide (NBS)
** ''N''-Iodosuccinimide (NIS)
* Trimethylsilyl
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom minus;Si(CH3)3 which is in turn bonded to the rest of a molecule. This structural group is ch ...
iodide (Me3SiI) in dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with w ...
at ambient temperature (selectivity can be achieved under specific conditions)
The ''p''-methoxybenzyl protecting group
''p''-Methoxybenzyl (PMB) is used as aprotecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
for alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
( 4-Methoxybenzylthiol is used to protect thiols).
 * Strong base such as powdered
* Strong base such as powdered potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploi ...
or sodium hydride and ''p''-methoxybenzyl halide (chloride or bromide)
* 4-methoxybenzyl-2,2,2-trichloroacetimidate can be used to install the PMB group in presence of:
** Scandium (III) triflate (Sc(OTf)3) in toluene at 0 °C
** Trifluoromethanesulfonic acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for este ...
(TfOH) in dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with w ...
at 0 °C
**: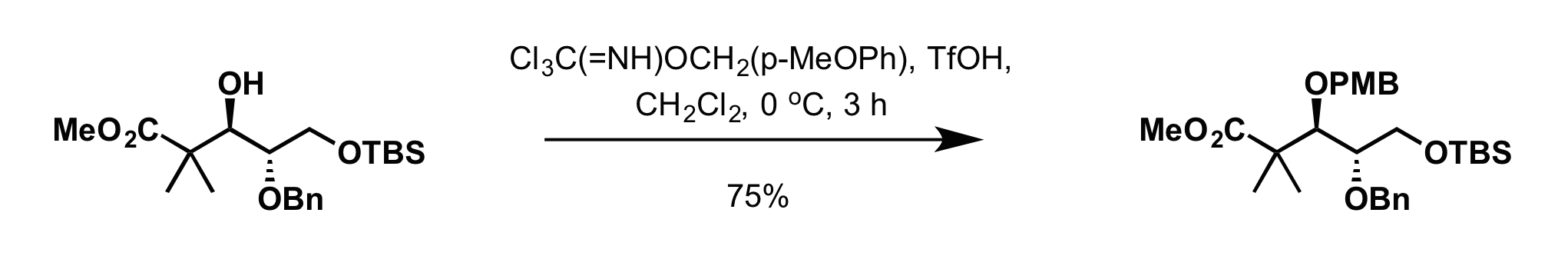
Deprotection methods
* 2,3-Dichloro-5,6-dicyano-''p''-benzoquinone (DDQ) *: * Conditions for deprotection of benzyl group are applicable for cleavage of the PMB protecting group
* Conditions for deprotection of benzyl group are applicable for cleavage of the PMB protecting group
Amine protection
The benzyl group is occasionally used as aprotecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
for amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent suc ...
s in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. Other methods exist.
* Aqueous potassium carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and ...
and benzyl halide ( BnCl, BnBr) in methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a lig ...
*: *
* Benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odo ...
, 6 M HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a s ...
and NaBH3CN in methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a lig ...
*:
Deprotection methods
*Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic c ...
in the presence of the palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
catalyst
See also
*Benzylamine
Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water ...
References
External links
* * {{functional group, state=expanded Aryl groups Protecting groups