|
Benzyl Chloride
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block. Preparation Benzyl chloride is prepared industrially by the gas-phase photochemical reaction of toluene with chlorine: :C6H5CH3 + Cl2 → C6H5CH2Cl + HCl In this way, approximately 100,000 tonnes are produced annually. The reaction proceeds by the free radical process, involving the intermediacy of free chlorine atoms. Side products of the reaction include benzal chloride and benzotrichloride. Other methods of production exist, such as the Blanc chloromethylation of benzene. Benzyl chloride was first prepared from treatment of benzyl alcohol with hydrochloric acid. Uses and reactions Industrially, benzyl chloride is the precursor to benzyl esters, which are used as plasticizers, flavorants, and perfumes. Phenylacetic acid, a precursor to pharmaceuticals, is produced from benzyl cyan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yellow
Yellow is the color between green and orange on the spectrum of light. It is evoked by light with a dominant wavelength of roughly 575585 nm. It is a primary color in subtractive color systems, used in painting or color printing. In the RGB color model, used to create colors on television and computer screens, yellow is a secondary color made by combining red and green at equal intensity. Carotenoids give the characteristic yellow color to autumn leaves, corn, canaries, daffodils, and lemons, as well as egg yolks, buttercups, and bananas. They absorb light energy and protect plants from photo damage in some cases. Sunlight has a slight yellowish hue when the Sun is near the horizon, due to atmospheric scattering of shorter wavelengths (green, blue, and violet). Because it was widely available, yellow ochre pigment was one of the first colors used in art; the Lascaux cave in France has a painting of a yellow horse 17,000 years old. Ochre and orpiment pigments were us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyanide
Sodium cyanide is a poisonous compound with the formula Na C N. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base. Production and chemical properties Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide: :HCN + NaOH → NaCN + H2O Worldwide production was estimated at 500,000 tons in the year 2006. Formerly it was prepared by the Castner process involving the reaction of sodium amide with carbon at elevated temperatures. : NaNH2 + C → NaCN + H2 The structure of solid NaCN is related to that of sodium chloride. The anions and cations are each six-coordinate. Potassium cyanide (KCN) adopts a similar structure. When treated with acid, it forms the toxic gas hydrogen cyanide: : NaCN + H+ → HCN + Na+ Because the salt is derived from a weak aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Cyanide
Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry. Preparation Benzyl cyanide can be produced via Kolbe nitrile synthesis between benzyl chloride and sodium cyanide and by oxidative decarboxylation of phenylalanine. Benzyl cyanides can also be prepared by arylation of silyl-substituted acetonitrile. Reactions Benzyl cyanide undergoes many reactions characteristic of nitriles. It can be hydrolyzed to give phenylacetic acid or it can be used in the Pinner reaction to yield phenylacetic acid esters. Hydrogenation gives β-phenethylamine. The compound contains an "active methylene unit". Bromination occurs gives PhCHBrCN. A variety of base-induced reactions result in the formation of new carbon-carbon bonds. Uses Benzyl cyanide is used as a solvent and as a starting material in the synthesis of fungicides (.e.g. Fenapanil), fragr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylacetic Acid
Phenylacetic acid (PAA; conjugate base phenylacetate), also known by various synonyms, is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a strong honey-like odor. Endogenously, it is a catabolite of phenylalanine. As a commercial chemical, because it can be used in the illicit production of phenylacetone (used in the manufacture of substituted amphetamines), it is subject to controls in countries including the United States and China. Occurrence Phenylacetic acid has been found to be an active auxin (a type of plant hormone), found predominantly in fruits. However, its effect is much weaker than the effect of the basic auxin molecule indole-3-acetic acid. In addition the molecule is naturally produced by the metapleural gland of most ant species and used as an antimicrobial. It is also the oxidation product of phenethylamine in humans following metabolism by monoamine oxidase and subsequent metabolism o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfume
Perfume (, ; french: parfum) is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory." Ancient texts and archaeological excavations show the use of perfumes in some of the earliest human civilizations. Modern perfumery began in the late 19th century with the commercial synthesis of aroma compounds such as vanillin or coumarin, which allowed for the composition of perfumes with smells previously unattainable solely from natural aromatics. History The word ''perfume'' derives from the Latin ''perfumare'', meaning "to smoke through". ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasticizer
A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture. Plasticizers are commonly added to polymers such as plastics and rubber, either to facilitate the handling of the raw material during fabrication, or to meet the demands of the end product's application. For example, plasticizers are commonly added to polyvinyl chloride (PVC), which otherwise is hard and brittle, to make it soft and pliable; which makes it suitable for products such as shower curtains, vinyl flooring, clothing, bags, flexible plastic tubing, and electric wire insulation/coating. Plasticizers are also often added to concrete formulations to make them more workable and fluid for pouring, thus allowing the water contents to be reduced. Similarly, they are often added to clays, stucco, solid rocket fuel, and other pastes prior t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precursor (chemistry)
In chemistry, a precursor is a compound that participates in a chemical reaction that produces another compound. In biochemistry, the term "precursor" often refers more specifically to a chemical compound preceding another in a metabolic pathway, such as a protein precursor. Illicit drug precursors In 1988, the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances introduced detailed provisions and requirements relating the control of precursors used to produce drugs of abuse. In Europe the Regulation (EC) No. 273/2004 of the European Parliament and of the Council on drug precursors was adopted on 11 February 2004. ( European law on drug precursors) Illicit explosives precursors On January 15, 2013, the Regulation (EU) No. 98/2013 of the European Parliament and of the Council on the marketing and use of explosives precursors was adopted. The Regulation harmonises rules across Europe on the making available, introduction, possession and u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl Alcohol
Benzyl alcohol is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is a useful solvent due to its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide. Natural occurrences Benzyl alcohol is produced naturally by many plants and is commonly found in fruits and teas. It is also found in a variety of essential oils including jasmine, hyacinth and ylang-ylang. It is also found in castoreum from the castor sacs of beavers. Benzyl esters also occur naturally. Preparation Benzyl alcohol is produced industrially from toluene via benzyl chloride, which is hydrolyzed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
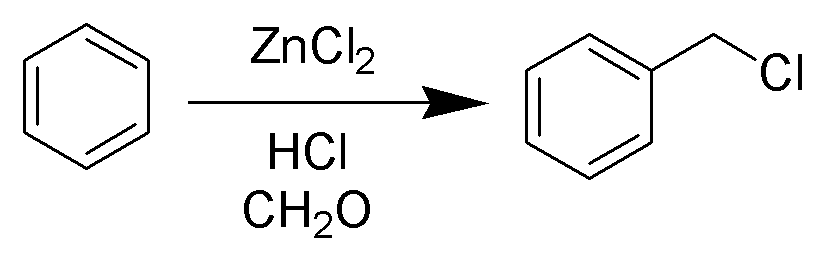
Blanc Chloromethylation
The Blanc chloromethylation (also called the Blanc reaction) is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923 Mechanism and scope The reaction is carried out under acidic conditions and with a ZnCl2 catalyst. These conditions protonate the formaldehyde carbonyl making the carbon much more electrophilic. The aldehyde is then attacked by the aromatic pi-electrons, followed by rearomatization of the aromatic ring. The benzyl alcohol thus formed is quickly converted to the chloride under the reaction conditions. Other possibilities for the electrophile include (chloromethyl)oxonium cation (ClH2C–OH2+) or chlorocarbenium cation (ClCH2+), which may be formed in the presence of zinc chloride. These species may account for the fact that moderately and strongly deactivated substrate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




2.png)