Amylose on:
[Wikipedia]
[Google]
[Amazon]
Amylose is a
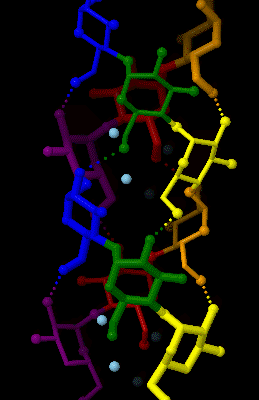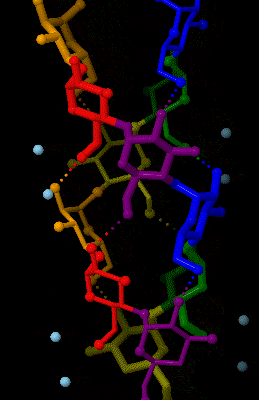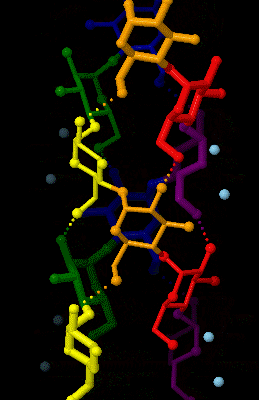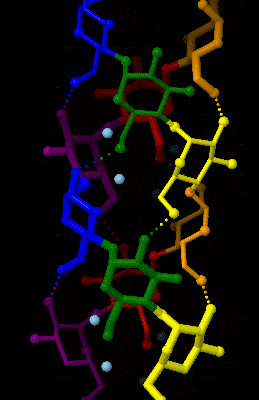 Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The
Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The
polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with w ...
made of α-D-glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
units, bonded to each other through α(1→4) glycosidic bonds
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
A glycosidic bond is formed between the hemiacetal or hemiketal gr ...
. It is one of the two components of starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human die ...
, making up approximately 20–30%. Because of its tightly packed helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch.
Structure
 Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The
Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula
The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bond ...
of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands.
There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix
A double is a look-alike or doppelgänger; one person or being that resembles another.
Double, The Double or Dubble may also refer to:
Film and television
* Double (filmmaking), someone who substitutes for the credited actor of a character
* ...
(A or B form), or it can bind with another hydrophobic guest molecule such as iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
, a fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, f ...
, or an aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
. This is known as the V form and is how amylopectin binds to amylose in the structure of starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human die ...
. Within this group, there are many different variations. Each is notated with V and then a subscript indicating the number of glucose units per turn. The most common is the V6 form, which has six glucose units a turn. V8 and possibly V7 forms exist as well. These provide an even larger space for the guest molecule to bind.
This linear structure can have some rotation around the phi and psi angles, but for the most part bound glucose ring oxygens lie on one side of the structure. The α(1→4) structure promotes the formation of a helix structure, making it possible for hydrogen bonds to form between the oxygen atoms bound at the 2-carbon of one glucose molecule and the 3-carbon of the next glucose molecule.
Fiber X-ray diffraction analysis coupled with computer-based structure refinement has found A-, B-, and C- polymorphs of amylose. Each form corresponds to either the A-, the B-, or the C- starch forms. A- and B- structures have different helical crystal structures and water contents, whereas the C- structure is a mixture of A- and B- unit cells, resulting in an intermediate packing density between the two forms.
Physical properties
Because the long linear chains of amylose more readily crystallize than amylopectin (which has short, highly branched chains), high-amylose starch is more resistant to digestion. Unlike amylopectin, amylose is not soluble in cold water. It also reduces the crystallinity of amylopectin and how easily water can infiltrate the starch. The higher the amylose content, the less expansion potential and the lower the gel strength for the same starch concentration. This can be countered partially by increasing the granule size.Function
Amylose is important in plant energy storage. It is less readily digested than amylopectin; however, because of its helical structure, it takes up less space compared to amylopectin. As a result, it is the preferred starch for storage in plants. It makes up about 30% of the stored starch in plants, though the specific percentage varies by species and variety. The digestive enzyme α-amylase is responsible for the breakdown of the starch molecule into maltotriose andmaltose
}
Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the tw ...
, which can be used as sources of energy.
Amylose is also an important thickener, water binder, emulsion stabilizer, and gelling agent in both industrial and food-based contexts. Loose helical amylose chains have a hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, ...
interior that can bind to hydrophobic molecules such as lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids in ...
and aromatic compounds. The one problem with this is that, when it crystallizes or associates, it can lose some stability, often releasing water in the process ( syneresis). When amylose concentration is increased, gel stickiness decreases but gel firmness increases. When other things including amylopectin bind to amylose, the viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the int ...
can be affected, but incorporating κ- carrageenan, alginate, xanthan gum, or low-molecular-weight sugars can reduce the loss in stability. The ability to bind water can add substance to food, possibly serving as a fat replacement. For example, amylose is responsible for causing white sauce to thicken, but, upon cooling, some separation between the solid and the water will occur. Amylose is known for its good film forming properties, hence carrying a potential importance in food packaging. Excellent film forming behavior of amylose was studied already in 1950s. Amylose films are better for both barrier properties and mechanical properties when compared to the amylopectin films.
In a laboratory setting, it can act as a marker. Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
molecules fit neatly inside the helical structure of amylose, binding with the starch polymer that absorbs certain known wavelengths of light. Hence, a common test is the iodine test
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a v ...
for starch. Mix starch with a small amount of yellow iodine solution. In the presence of amylose, a blue-black color will be observed. The intensity of the color can be tested with a colorimeter, using a red filter to discern the concentration of starch present in the solution. It is also possible to use starch as an indicator in titrations involving iodine reduction. It is also used in amylose magnetic beads and resin to separate maltose-binding protein
Maltose-binding protein (MBP) is a part of the maltose/maltodextrin system of ''Escherichia coli'', which is responsible for the uptake and efficient catabolism of maltodextrins. It is a complex regulatory and transport system involving many prot ...
Recent studies
High-amylose varieties ofrice
Rice is the seed of the grass species '' Oryza sativa'' (Asian rice) or less commonly ''Oryza glaberrima'' (African rice). The name wild rice is usually used for species of the genera '' Zizania'' and '' Porteresia'', both wild and domesticat ...
, the less sticky long-grain rice, have a much lower glycemic load, which could be beneficial for diabetics.
Researchers have identified the Granule Bound Starch Synthase (GBSS) as the enzyme that specifically elongates amylose during starch biosynthesis in plants. The waxy locus in maize encodes for the GBSS protein. Mutants lacking the GBSS protein produce starch containing only amylopectin, such as in waxy corn. In Arabidopsis leaves, another gene, encoding the Protein Targeting to STarch (PTST) protein, is required in addition to GBSS for amylose synthesis. Mutants lacking either protein produce starch without amylose. Genetically modified potato cultivar Amflora by BASF Plant Science was developed to not produce amylose.
See also
* Amflora, genetically modified low amylose potato (high in amylopectin) *Amylomaize Amylomaize was a term coined in the late 1940s by Robert P. Bear of Bear Hybrids Corn Company in Decatur, Illinois to describe his discovery and commercial breeding of a cornstarch with high (>50%) amylose content, also called high amylose starch. ...
, high amylose maize starch
* Russet Burbank potato, high amylose potato cultivar
References
External links
* {{Authority control Polysaccharides Starch