Litmus Paper on:
[Wikipedia]
[Google]
[Amazon]

 Litmus is a water-soluble mixture of different
Litmus is a water-soluble mixture of different

dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution an ...
s extract
An extract is a substance made by extracting a part of a raw material, often by using a solvent such as ethanol, oil or water. Extracts may be sold as tinctures, absolutes or in powder form.
The aromatic principles of many spices, nuts, h ...
ed from lichen
A lichen ( , ) is a composite organism that arises from algae or cyanobacteria living among filaments of multiple fungi species in a mutualistic relationship.filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity. It is a purple
dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution an ...
that is extracted from a type of algal bloom called ‘lichens’. In an acidic medium, blue litmus paper turns red, and red litmus paper turns blue in a basic or alkaline medium.
History
The word "litmus" comes from an Old Norse word for "pulp". About 1300 the Spanish physician Arnaldus de Villa Nova began using litmus to study acids and bases. From the 16th century onwards, the blue dye was extracted from somelichen
A lichen ( , ) is a composite organism that arises from algae or cyanobacteria living among filaments of multiple fungi species in a mutualistic relationship.Netherlands.
 Litmus can be found in different species of lichens. The dyes are extracted from such species as '' Roccella tinctoria'' (South American), '' Roccella fuciformis'' (Angola and Madagascar), '' Roccella pygmaea'' (Algeria), '' Roccella phycopsis'', ''
Litmus can be found in different species of lichens. The dyes are extracted from such species as '' Roccella tinctoria'' (South American), '' Roccella fuciformis'' (Angola and Madagascar), '' Roccella pygmaea'' (Algeria), '' Roccella phycopsis'', ''
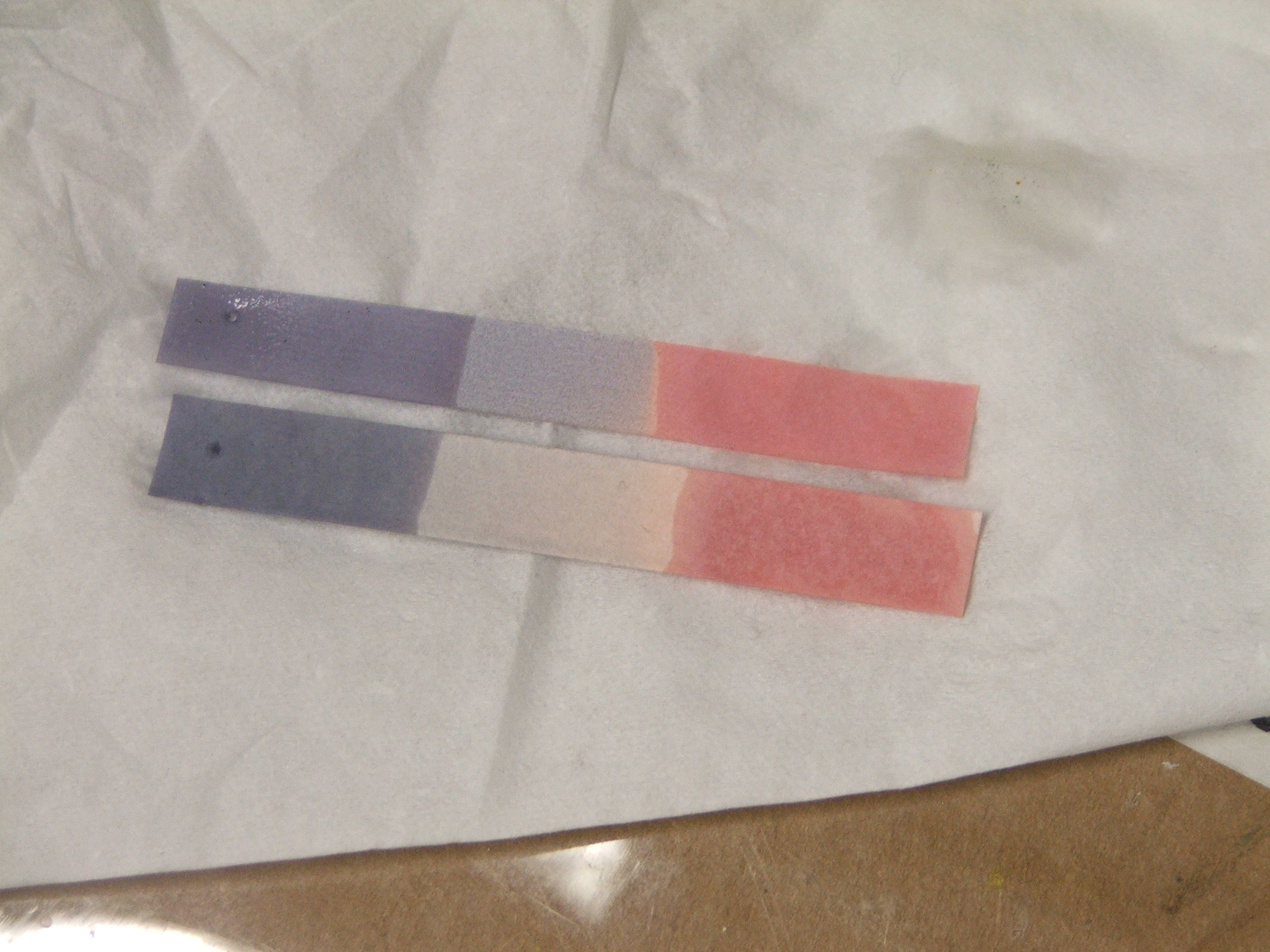 The main use of litmus is to test whether a solution is acidic or
The main use of litmus is to test whether a solution is acidic or
Natural sources
 Litmus can be found in different species of lichens. The dyes are extracted from such species as '' Roccella tinctoria'' (South American), '' Roccella fuciformis'' (Angola and Madagascar), '' Roccella pygmaea'' (Algeria), '' Roccella phycopsis'', ''
Litmus can be found in different species of lichens. The dyes are extracted from such species as '' Roccella tinctoria'' (South American), '' Roccella fuciformis'' (Angola and Madagascar), '' Roccella pygmaea'' (Algeria), '' Roccella phycopsis'', ''Lecanora tartarea
''Lecanora'' is a genus of lichen commonly called rim lichens.Field Guide to California Lichens, Stephen Sharnoff, Yale University Press, 2014, Lichens in the genus ''Squamarina'' are also called rim lichens. Members of the genus have roughly ci ...
'' (Norway, Sweden), ''Variolaria dealbata'', '' Ochrolechia parella'', '' Parmotrema tinctorum'', and '' Parmelia''. Currently, the main sources are '' Roccella montagnei'' (Mozambique) and '' Dendrographa leucophoea'' (California).
Uses
basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
, as blue litmus paper turns red under acidic conditions, and red litmus paper turns blue under basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
or alkaline
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a base (chemistry), basic, ionic compound, ionic salt (chemistry), salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as ...
conditions, with the color change occurring over the pH range 4.5–8.3 at . Neutral litmus paper is purple. Wet litmus paper can also be used to test for water-soluble gases that affect acidity or basicity
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rou ...
; the gas dissolves in the water and the resulting solution colors the litmus paper. For instance, ammonia gas, which is alkaline, turns red litmus paper blue. While all litmus paper acts as pH paper, the opposite is not true.
Litmus can also be prepared as an aqueous solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be re ...
that functions similarly. Under acidic conditions, the solution is red, and under alkaline conditions, the solution is blue.
Chemical reactions other than acid–base can also cause a color change to litmus paper. For instance, chlorine gas turns blue litmus paper white; the litmus dye is bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to ...
ed because hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of ble ...
ions are present. This reaction is irreversible, so the litmus is not acting as an indicator in this situation.
Chemistry
The litmus mixture has the CAS number 1393-92-6 and contains 10 to around 15 different dyes. All of the chemical components of litmus are likely to be the same as those of the related mixture known as orcein, but in different proportions. In contrast with orcein, the principal constituent of litmus has an average molecular mass of 3300. Acid-base indicators on litmus owe their properties to a 7-hydroxyphenoxazonechromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
. Some fractions of litmus were given specific names including erythrolitmin Erythrolitmin (also called erythrolein) is the active ingredient extracted from the Litmus lichen, used in chemistry as a pH indicator. The erythrolitmin molecule is related to the orceins, and consists essentially of several phenoxazone and orcin ...
(or erythrolein), azolitmin, spaniolitmin, leucoorcein, and leucazolitmin. Azolitmin shows nearly the same effect as litmus.
A recipe to make litmus out of the lichens, as outlined on a UC Santa Barbara website says:
Details are difficult to find because the processes were kept secret.
This summary of a modern manufacturing procedure is from The vanishing lichens, D H S Richardson, London, 1975.
The lichens (preferably Lecanora tartarea
''Lecanora'' is a genus of lichen commonly called rim lichens.Field Guide to California Lichens, Stephen Sharnoff, Yale University Press, 2014, Lichens in the genus ''Squamarina'' are also called rim lichens. Members of the genus have roughly ci ...
and Roccella tinctoria) are ground in a solution of sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
and ammonia.
Stir the lichens from time to time and the color changes from red to purple and finally blue after about four weeks. The lichens are then dried and powdered. At this stage the lichens contain partly litmus and partly orcein pigments. The orcein is removed by extraction with alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
, leaving the pure blue litmus. It is marketed as blue lumps, masses, or tablets, after mixing with colorless compounds such as chalk and gypsum. Litmus paper is paper impregnated with this substance.
Mechanism
Red litmus contains a weak diprotic acid. When it is exposed to a basic compound, the hydrogen ions react with the added base. The conjugate base formed from the litmus acid has a blue color, so the wet red litmus paper turns blue in alkaline solution.References
{{reflist PH indicators Paper products