zinc sulfide on:
[Wikipedia]
[Google]
[Amazon]
Zinc sulfide (or zinc sulphide) is an
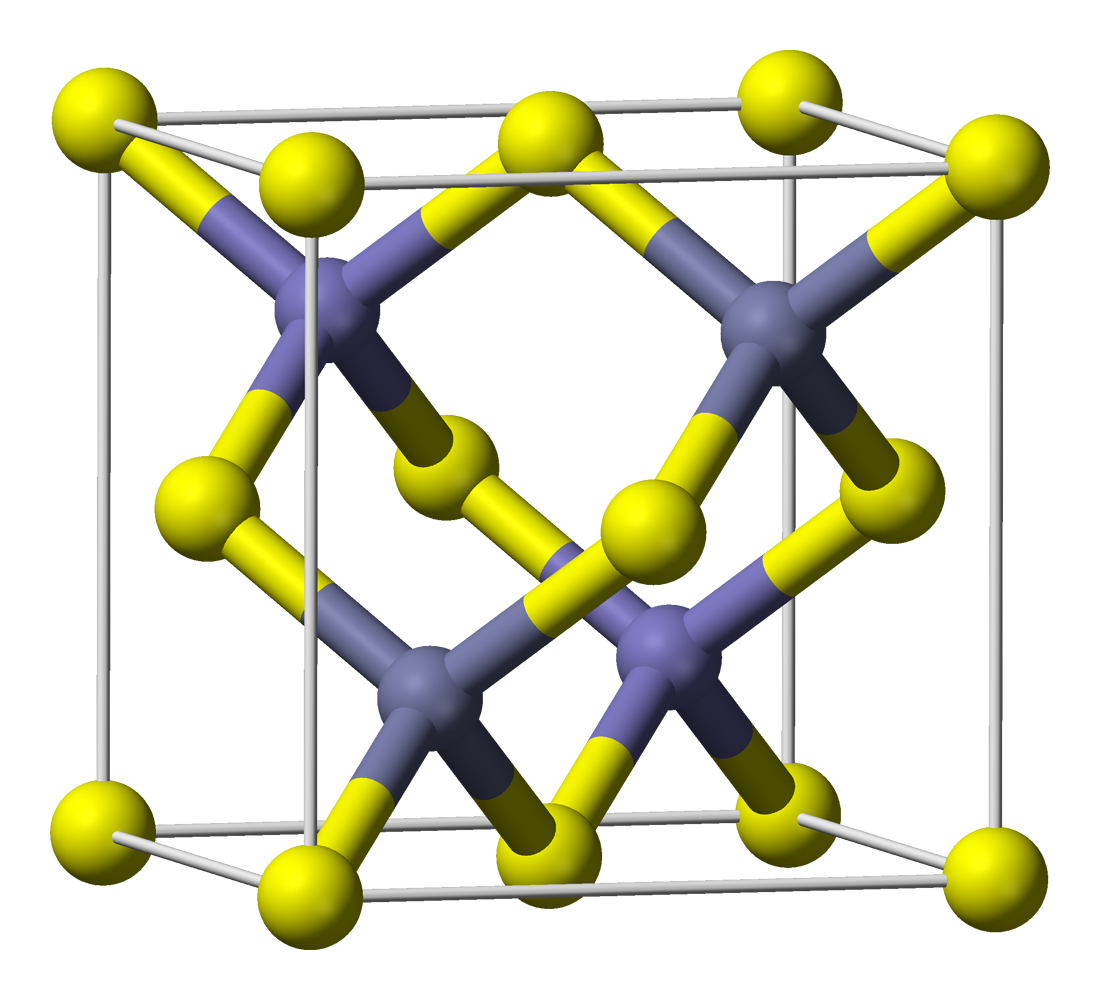
 ZnS exists in two main
ZnS exists in two main
inorganic compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemi ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Va ...
. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from aroun ...
optics.
Structure

 ZnS exists in two main
ZnS exists in two main crystalline form
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns t ...
s. This dualism is an example of polymorphism
Polymorphism, polymorphic, polymorph, polymorphous, or polymorphy may refer to:
Computing
* Polymorphism (computer science), the ability in programming to present the same programming interface for differing underlying forms
* Ad hoc polymorphis ...
. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Va ...
. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The d ...
. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula .
Applications
Luminescent material
Zinc sulfide, with addition of few ppm of suitable activator, exhibits strong phosphorescence. The phenomenon was described byNikola Tesla
Nikola Tesla ( ; ,"Tesla"
'' cathode ray tubes through
 Zinc sulfide is usually produced from waste materials from other applications. Typical sources include smelter, slag, and pickle liquors. As an example- the synthesis of
Zinc sulfide is usually produced from waste materials from other applications. Typical sources include smelter, slag, and pickle liquors. As an example- the synthesis of
Zinc and Sulfur
at '' The Periodic Table of Videos'' (University of Nottingham)
Composition of CRT phosphors
University of Reading, Infrared Multilayer Laboratory
optical data
melting point {{Sulfides Sulfides
'' cathode ray tubes through
X-ray
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ' ...
screens to glow in the dark products. When silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
is used as activator, the resulting color is bright blue, with maximum at 450 nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, ...
s. Using manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy u ...
yields an orange-red color at around 590 nanometers. Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
gives long-time glow, and it has the familiar greenish glow-in-the-dark. Copper-doped zinc sulfide ("ZnS plus Cu") is used also in electroluminescent panels. It also exhibits phosphorescence due to impurities on illumination with blue or ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiati ...
light.
Optical material
Zinc sulfide is also used as aninfrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from aroun ...
optical material, transmitting from visible wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
s to just over 12 micrometer Micrometer can mean:
* Micrometer (device), used for accurate measurements by means of a calibrated screw
* American spelling of micrometre
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; ...
s. It can be used planar as an optical window or shaped into a lens
A lens is a transmissive optical device which focuses or disperses a light beam by means of refraction. A simple lens consists of a single piece of transparent material, while a compound lens consists of several simple lenses (''elements'' ...
. It is made as microcrystalline sheets by the synthesis from hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
gas and zinc vapour, and this is sold as FLIR-grade (Forward Looking Infrared), where the zinc sulfide is in a milky-yellow, opaque form. This material when hot isostatically pressed (HIPed) can be converted to a water-clear form known as Cleartran (trademark). Early commercial forms were marketed as Irtran-2 but this designation is now obsolete.
Pigment
Zinc sulfide is a commonpigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic comp ...
, sometimes called sachtolith. When combined with barium sulfate, zinc sulfide forms lithopone.Gerhard Auer, Peter Woditsch, Axel Westerhaus, Jürgen Kischkewitz, Wolf-Dieter Griebler and Marcel Liedekerke "Pigments, Inorganic, 2. White Pigments" in Ullmann's Encyclopedia of Industrial Chemistry 2009, Wiley-VCH, Weinheim.
Catalyst
Fine ZnS powder is an efficient photocatalyst, which produces hydrogen gas from water upon illumination. Sulfur vacancies can be introduced in ZnS during its synthesis; this gradually turns the white-yellowish ZnS into a brown powder, and boosts the photocatalytic activity through enhanced light absorption.Semiconductor properties
Both sphalerite and wurtzite are intrinsic, wide- bandgapsemiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
s. These are prototypical II-VI semiconductors, and they adopt structures related to many of the other semiconductors, such as gallium arsenide. The cubic form of ZnS has a band gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference ( ...
of about 3.54 electron volts at 300 kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and ph ...
s, but the hexagonal form has a band gap of about 3.91 electron volts. ZnS can be doped as either an n-type semiconductor or a p-type semiconductor.
History
The phosphorescence of ZnS was first reported by the French chemistThéodore Sidot
Théodore Sidot was a French chemist who, in 1866, discovered the phosphorescence of zinc sulphide. He worked at the Lycée Charlemagne in Paris, as chemistry preparator. He was injured in the 1870 Franco-Prussian War at the Fort de Nogent. He rec ...
in 1866. His findings were presented by A. E. Becquerel, who was renowned for the research on luminescence. ZnS was used by Ernest Rutherford and others in the early years of nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies the ...
as a scintillation
Scintillation can refer to:
*Scintillation (astronomy), atmospheric effects which influence astronomical observations
*Interplanetary scintillation, fluctuations of radio waves caused by the solar wind
*Scintillation (physics), a flash of light pro ...
detector, because it emits light upon excitation by x-rays
X-rays (or rarely, ''X-radiation'') are a form of high-energy electromagnetic radiation. In many languages, it is referred to as Röntgen radiation, after the German scientist Wilhelm Conrad Röntgen, who discovered it in 1895 and named it ' ...
or electron beam, making it useful for X-ray screens and cathode ray tubes. This property made zinc sulfide useful in the dials of radium watches.
Production
 Zinc sulfide is usually produced from waste materials from other applications. Typical sources include smelter, slag, and pickle liquors. As an example- the synthesis of
Zinc sulfide is usually produced from waste materials from other applications. Typical sources include smelter, slag, and pickle liquors. As an example- the synthesis of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogeno ...
from methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
requires a priori removal of hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The und ...
impurities in the natural gas, for which zinc oxide
Zinc oxide is an inorganic compound with the Chemical formula, formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceram ...
is used. This scavenging produces zinc sulfide:
:ZnO + H2S → ZnS + H2O
Laboratory preparation
It is easily produced by igniting a mixture ofzinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
. Since zinc sulfide is insoluble in water, it can also be produced in a precipitation reaction. Solutions containing Zn2+ salts readily form a precipitate ZnS in the presence of sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds l ...
ions (e.g., from H2S).
:Zn2+ + S2− → ZnS
This reaction is the basis of a gravimetric analysis
Gravimetric analysis describes a set of methods used in analytical chemistry
Analytical chemistry studies and uses instruments and methods to separate, identify, and quantify matter. In practice, separation, identification or quantificati ...
for zinc.
References
External links
Zinc and Sulfur
at '' The Periodic Table of Videos'' (University of Nottingham)
Composition of CRT phosphors
University of Reading, Infrared Multilayer Laboratory
optical data
melting point {{Sulfides Sulfides
sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds l ...
II-VI semiconductors
Luminescence
Optical materials
Phosphors and scintillators
*
Zincblende crystal structure
Wurtzite structure type