Zeotrope on:
[Wikipedia]
[Google]
[Amazon]
A zeotropic mixture, or non-azeotropic mixture, is a mixture with liquid components that have different
 In mixtures of substances, the bubble point is the saturated liquid temperature, whereas the saturated vapor temperature is called the dew point. Because the bubble and dew lines of a zeotropic mixture's temperature-composition diagram do not intersect, a zeotropic mixture in its liquid phase has a different fraction of a component than the gas phase of the mixture. On a temperature-composition diagram, after a mixture in its liquid phase is heated to the temperature at the bubble (boiling) curve, the fraction of a component in the mixture changes along an isothermal line connecting the dew curve to the boiling curve as the mixture boils. At any given temperature, the composition of the liquid is the composition at the bubble point, whereas the composition of the vapor is the composition at the dew point. Unlike azeotropic mixtures, there is no azeotropic point at any temperature on the diagram where the bubble line and dew lines would intersect. Thus, the composition of the mixture will always change between the bubble and dew point component fractions upon boiling from a liquid to a gas until the mass fraction of a component reaches 1 (i.e. the zeotropic mixture is completely separated into its pure components). As shown in Figure 1, the mole fraction of component 1 decreases from 0.4 to around 0.15 as the liquid mixture boils to the gas phase.
In mixtures of substances, the bubble point is the saturated liquid temperature, whereas the saturated vapor temperature is called the dew point. Because the bubble and dew lines of a zeotropic mixture's temperature-composition diagram do not intersect, a zeotropic mixture in its liquid phase has a different fraction of a component than the gas phase of the mixture. On a temperature-composition diagram, after a mixture in its liquid phase is heated to the temperature at the bubble (boiling) curve, the fraction of a component in the mixture changes along an isothermal line connecting the dew curve to the boiling curve as the mixture boils. At any given temperature, the composition of the liquid is the composition at the bubble point, whereas the composition of the vapor is the composition at the dew point. Unlike azeotropic mixtures, there is no azeotropic point at any temperature on the diagram where the bubble line and dew lines would intersect. Thus, the composition of the mixture will always change between the bubble and dew point component fractions upon boiling from a liquid to a gas until the mass fraction of a component reaches 1 (i.e. the zeotropic mixture is completely separated into its pure components). As shown in Figure 1, the mole fraction of component 1 decreases from 0.4 to around 0.15 as the liquid mixture boils to the gas phase.
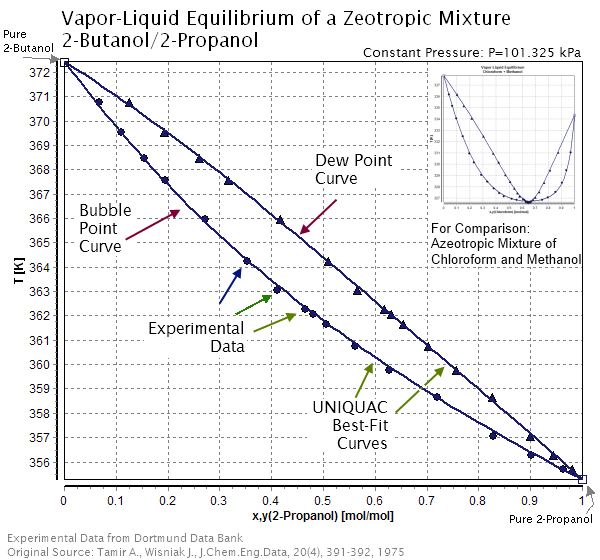 Azeotropic and zeotropic mixtures have different dew and bubble curves characteristics in a temperature-composition graph. Namely, azeotropic mixtures have dew and bubble curves that intersect, but zeotropic mixtures do not. In other words, zeotropic mixtures have no azeotropic points. An azeotropic mixture that is near its azeotropic point has negligible zeotropic behavior and is near-azeotropic rather than zeotropic.
Zeotropic mixtures differ from azeotropic mixtures in that the vapor and liquid phases of an azeotropic mixture have the same fraction of constituents. This is due to the constant boiling point of the azeotropic mixture.
Azeotropic and zeotropic mixtures have different dew and bubble curves characteristics in a temperature-composition graph. Namely, azeotropic mixtures have dew and bubble curves that intersect, but zeotropic mixtures do not. In other words, zeotropic mixtures have no azeotropic points. An azeotropic mixture that is near its azeotropic point has negligible zeotropic behavior and is near-azeotropic rather than zeotropic.
Zeotropic mixtures differ from azeotropic mixtures in that the vapor and liquid phases of an azeotropic mixture have the same fraction of constituents. This is due to the constant boiling point of the azeotropic mixture.
 The ideal case of distillation uses zeotropic mixtures. Zeotropic fluid and gaseous mixtures can be separated by
The ideal case of distillation uses zeotropic mixtures. Zeotropic fluid and gaseous mixtures can be separated by
boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
s. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidi ...
or condense
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor to ...
at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic State of ...
occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will cond ...
. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
Zeotropic mixtures have different characteristics in nucleate and convective boiling, as well as in the organic Rankine cycle
In thermal engineering, the Organic Rankine Cycle (ORC) is a type of thermodynamic cycle. It is a variation of the Rankine cycle named for its use of an organic, high-molecular-mass fluid whose vaporization temperature is lower than that of wat ...
. Because zeotropic mixtures have different properties than pure fluids or azeotropic mixtures, zeotropic mixtures have many unique applications in industry, namely in distillation, refrigeration, and cleaning processes.
Dew and bubble points
Temperature glides
Different zeotropic mixtures have different temperature glides. For example, zeotropic mixture R152a/R245fa has a higher temperature glide than R21/R245fa. A larger gap between the boiling points creates a larger temperature glide between the boiling curve and dew curve at a given mass fraction. However, with any zeotropic mixture, the temperature glide decreases when the mass fraction of a component approaches 1 or 0 (i.e. when the mixture is almost separated into its pure components) because the boiling and dew curves get closer near these mass fractions. A larger difference in boiling points between the substances also affects the dew and bubble curves of the graph. A larger difference in boiling points creates a larger shift in mass fractions when the mixture boils at a given temperature.Zeotropic vs. azeotropic mixtures
 Azeotropic and zeotropic mixtures have different dew and bubble curves characteristics in a temperature-composition graph. Namely, azeotropic mixtures have dew and bubble curves that intersect, but zeotropic mixtures do not. In other words, zeotropic mixtures have no azeotropic points. An azeotropic mixture that is near its azeotropic point has negligible zeotropic behavior and is near-azeotropic rather than zeotropic.
Zeotropic mixtures differ from azeotropic mixtures in that the vapor and liquid phases of an azeotropic mixture have the same fraction of constituents. This is due to the constant boiling point of the azeotropic mixture.
Azeotropic and zeotropic mixtures have different dew and bubble curves characteristics in a temperature-composition graph. Namely, azeotropic mixtures have dew and bubble curves that intersect, but zeotropic mixtures do not. In other words, zeotropic mixtures have no azeotropic points. An azeotropic mixture that is near its azeotropic point has negligible zeotropic behavior and is near-azeotropic rather than zeotropic.
Zeotropic mixtures differ from azeotropic mixtures in that the vapor and liquid phases of an azeotropic mixture have the same fraction of constituents. This is due to the constant boiling point of the azeotropic mixture.
Boiling
Whensuperheating
In thermodynamics, superheating (sometimes referred to as boiling retardation, or boiling delay) is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling. This is a so-called ''metastable state ...
a substance, nucleate pool boiling and convective flow boiling occur when the temperature of the surface used to heat a liquid is higher than the liquid's boiling point by the wall superheat.
Nucleate pool boiling
The characteristics of pool boiling are different for zeotropic mixtures than that of pure mixtures. For example, the minimum superheating needed to achieve this boiling is greater for zeotropic mixtures than for pure liquids because of the different proportions of individual substances in the liquid versus gas phases of the zeotropic mixture. Zeotropic mixtures and pure liquids also have different critical heat fluxes. In addition, theheat transfer coefficient
In thermodynamics, the heat transfer coefficient or film coefficient, or film effectiveness, is the proportionality constant between the heat flux and the thermodynamic driving force for the flow of heat (i.e., the temperature difference, ). ...
s of zeotropic mixtures are less than the ideal values predicted using the coefficients of pure liquids. This decrease in heat transfer is due to the fact that the heat transfer coefficients of zeotropic mixtures do not increase proportionately with the mass fractions of the mixture's components.
Convective flow boiling
Zeotropic mixtures have different characteristics in convective boiling than pure substances or azeotropic mixtures. Overall, zeotropic mixtures transfer heat more efficiently at the bottom of the fluid, whereas pure and azeotropic substances transfer heat better at the top. During convective flow boiling, the thickness of the liquid film is less at the top of the film than at the bottom because of gravity. In the case of pure liquids and azeotropic mixtures, this decrease in thickness causes a decrease in the resistance to heat transfer. Thus, more heat is transferred and the heat transfer coefficient is higher at the top of the film. The opposite occurs for zeotropic mixtures. The decrease in film thickness near the top causes the component in the mixture with the higher boiling point to decrease in mass fraction. Thus, the resistance to mass transfer increases near the top of the liquid. Less heat is transferred, and the heat transfer coefficient is lower than at the bottom of the liquid film. Because the bottom of the liquid transfers heat better, it requires a lower wall temperature near the bottom than at the top to boil the zeotropic mixture.Heat transfer coefficient
From low cryogenic to room temperatures, the heat transfer coefficients of zeotropic mixtures are sensitive to the mixture's composition, the diameter of the boiling tube, heat and mass fluxes, and the roughness of the surface. In addition, diluting the zeotropic mixture reduces the heat transfer coefficient. Decreasing the pressure when boiling the mixture only increases the coefficient slightly. Using grooved rather than smooth boiling tubes increases the heat transfer coefficient.Distillation
distillation
Distillation, or classical distillation, is the process of separation process, separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distilla ...
due to the difference in boiling points between the component mixtures. This process involves the use of vertically-arranged distillation columns (see Figure 2).
Distillation columns
When separating zeotropic mixtures with three or greater liquid components, each distillation column removes only the lowest-boiling point component and the highest boiling point component. In other words, each column separates two components purely. If three substances are separated with a single column, the substance with the intermediate boiling point will not be purely separated, and a second column would be needed. To separate mixtures consisting of multiple substances, a sequence of distillation columns must be used. This multi-step distillation process is also called rectification. In each distillation column, pure components form at the top (rectifying section) and bottom (stripping section) of the column when the starting liquid (called feed composition) is released in the middle of the column. This is shown in Figure 2. At a certain temperature, the component with the lowest boiling point (called distillate or overhead fraction) vaporizes and collects at the top of the column, whereas the component with the highest boiling point (called bottoms or bottom fraction) collects at the bottom of the column. In a zeotropic mixture, where more than one component exists, individual components move relative to each other as vapor flows up and liquid falls down. The separation of mixtures can be seen in a concentration profile. In a concentration profile, the position of a vapor in the distillation column is plotted against the concentration of the vapor. The component with the highest boiling point has a max concentration at the bottom of the column, where the component with the lowest boiling point has a max concentration at the top of the column. The component with the intermediate boiling point has a max concentration in the middle of the distillation column. Because of how these mixtures separate, mixtures with greater than three substances require more than one distillation column to separate the components.Distillation configurations
Many configurations can be used to separate mixtures into the same products, though some schemes are more efficient, and different column sequencings are used to achieve different needs. For example, a zeotropic mixture ABC can be first separated into A and BC before separating BC to B and C. On the other hand, mixture ABC can be first separated into AB and C, and AB can lastly be separated into A and B. These two configurations are sharp-split configurations in which the intermediate boiling substance does not contaminate each separation step. On the other hand, the mixture ABC could first be separated into AB and BC, and lastly split into A, B, and C in the same column. This is a non-sharp split configuration in which the substance with the intermediate boiling point is present in different mixtures after a separation step.Efficiency optimization
When designing distillation processes for separating zeotropic mixtures, the sequencing of distillation columns is vital to saving energy and costs. In addition, other methods can be used to lower the energy or equipment costs required to distill zeotropic mixtures. This includes combining distillation columns, using side columns, combining main columns with side columns, and re-usingwaste heat
Waste heat is heat that is produced by a machine, or other process that uses energy, as a byproduct of doing work. All such processes give off some waste heat as a fundamental result of the laws of thermodynamics. Waste heat has lower utility ...
for the system. After combining distillation columns, the amount of energy used is only that of one separated column rather than both columns combined. In addition, using side columns saves energy by preventing different columns from carrying out the same separation of mixtures. Combining main and side columns saves equipment costs by reducing the number of heat exchangers in the system. Re-using waste heat requires the amount of heat and temperature levels of the waste to match that of the heat needed. Thus, using waste heat requires changing the pressure inside evaporator
An evaporator is a device used to turn the liquid form of a chemical substance, such as water, into a vapor.
Uses
Air conditioning and refrigeration
Some air conditioners and refrigerators use a compressed liquid with a low boiling point, ...
s and condensers __NOTOC__
Condenser may refer to:
Heat transfer
* Condenser (heat transfer), a device or unit used to condense vapor into liquid. Specific types include:
** HVAC air coils
** Condenser (laboratory), a range of laboratory glassware used to remove ...
of the distillation system in order to control the temperatures needed. Controlling the temperature levels in a part of a system is possible with Pinch Technology. These energy-saving techniques have a wide application in industrial distillation of zeotropic mixtures: side columns have been used to refine crude oil
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crude ...
, and combining main and side columns is increasingly used.
Examples of zeotropic mixtures
Examples of distillation for zeotropic mixtures can be found in industry. Refining crude oil is an example of multi-component distillation in industry that has been used for more than 75 years. Crude oil is separated into five components with main and side columns in a sharp split configuration. In addition, ethylene is separated from methane and ethane for industrial purposes using multi-component distillation. Separating aromatic substances requires extractive distillation, for example, distilling a zeotropic mixture of benzene, toluene, and p-xylene.Refrigeration
Zeotropic mixtures that are used in refrigeration are assigned a number in the 400 series to help identify its component and their proportions as a part of nomenclature. Whereas for azeotropic mixtures they are assigned a number in the 500 series. According toASHRAE
The American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE ) is an American professional association seeking to advance heating, ventilation, air conditioning and refrigeration (HVAC&R) systems design and constructio ...
, refrigerants names start with 'R' followed by a series of numbers—400 series if it is zeotropic or 500 if it is azeotropic—followed by uppercase letters that denote the composition.
Research has proposed using zeotropic mixtures as substitutes to halogenated refrigerant
A refrigerant is a working fluid used in the heat pump and refrigeration cycle, refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Ref ...
s due to the harmful effects that hydrochlorofluorocarbons (HCFC) and chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and prop ...
s (CFC) have on the ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in rela ...
and global warming
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to E ...
. Researchers have focused on using new mixtures that have the same properties as past refrigerants to phase out harmful halogenated substances, in accordance to the Montreal Protocol
The Montreal Protocol is an international treaty designed to protect the ozone layer by phasing out the production of numerous substances that are responsible for ozone depletion
Ozone depletion consists of two related events observed sinc ...
and Kyoto Protocol
The Kyoto Protocol was an international treaty which extended the 1992 United Nations Framework Convention on Climate Change (UNFCCC) that commits state parties to reduce greenhouse gas emissions, based on the scientific consensus that (part ...
. For example, researchers found that zeotropic mixture R-404A can replace R-12, a CFC, in household refrigerators. However, there are some technical difficulties for using zeotropic mixtures. This includes leakages, as well as the high temperature glide associated with substances of different boiling points, though the temperature glide can be matched to the temperature difference between the two refrigerants when exchanging heat to increase efficiency. Replacing pure refrigerants with mixtures calls for more research on the environmental impact as well as the flammability and safety of refrigerant mixtures.
Organic Rankine cycle
In theOrganic Rankine Cycle
In thermal engineering, the Organic Rankine Cycle (ORC) is a type of thermodynamic cycle. It is a variation of the Rankine cycle named for its use of an organic, high-molecular-mass fluid whose vaporization temperature is lower than that of wat ...
(ORC), zeotropic mixtures are more thermally efficient than pure fluids. Due to their higher boiling points, zeotropic working fluid
For fluid power, a working fluid is a gas or liquid that primarily transfers force, motion, or mechanical energy. In hydraulics, water or hydraulic fluid transfers force between hydraulic components such as hydraulic pumps, hydraulic cylinders, a ...
s have higher net outputs of energy at the low temperatures of the Rankine Cycle than pure substances. Zeotropic working fluids condense across a range of temperatures, allowing external heat exchangers to recover the heat of condensation as a heat source for the Rankine Cycle. The changing temperature of the zeotropic working fluid can be matched to that of the fluid being heated or cooled to save waste heat because the mixture's evaporation process occurs at a temperature glide (see Pinch Analysis
Pinch analysis is a methodology for minimising energy consumption of process (engineering), chemical processes by calculating thermodynamically feasible ''energy targets'' (or minimum energy consumption) and achieving them by optimising heat reco ...
).
R21/R245fa and R152a/R245fa are two examples of zeotropic working fluids that can absorb more heat than pure R245fa due to their increased boiling points. The power output increases with the proportion of R152a in R152a/R245fa. R21/R245fa uses less heat and energy than R245fa. Overall, zeotropic mixture R21/R245fa has better thermodynamic properties than pure R245fa and R152a/R245fa as a working fluid in the ORC.
Cleaning processes
Zeotropic mixtures can be used as solvents in cleaning processes in manufacturing. Cleaning processes that use zeotropic mixtures include cosolvent processes and bisolvent processes.Cosolvent and bisolvent processes
In a cosolvent system, two miscible fluids with different boiling points are mixed to create a zeotropic mixture. The first fluid is a solvating agent that dissolves soil in the cleaning process. This fluid is an organic solvent with a low-boiling point and a flash point greater than the system's operating temperature. After the solvent mixes with the oil, the second fluid, a hydrofluoroether rinsing agent (HFE), rinses off the solvating agent. The solvating agent can be flammable because its mixture with the HFE is nonflammable. In bisolvent cleaning processes, the rinsing agent is separated from the solvating agent. This makes the solvating and rinsing agents more effective because they are not diluted. Cosolvent systems are used for heavy oils, waxes, greases and fingerprints, and can remove heavier soils than processes that use pure or azeotropic solvents. Cosolvent systems are flexible in that different proportions of substances in the zeotropic mixture can be used to satisfy different cleaning purposes. For example, increasing the proportion of solvating agent to rinsing agent in the mixture increases the solvency, and thus is used for removing heavier soils. The operating temperature of the system depends on the boiling point of the mixture, which in turn depends on the compositions of these agents in zeotropic mixture. Since zeotropic mixtures have different boiling points, the cleaning and rinse sump have different ratios of cleaning and solvating agents. The lower-boiling point solvating agent is not found in the rinse sump due to the large difference in boiling points between the agents.Examples of zeotropic solvents
Mixtures containing HFC-43-10mee can replace CFC-113 and perfluorocarbon (PFC) as solvents in cleaning systems because HFC-43-10mee does not harm the ozone layer, unlike CFC-113 and PFC. Various mixtures of HFC-43-10mee are commercially available for a variety of cleaning purposes. Examples of zeotropic solvents in cleaning processes include: * Zeotropic mixtures of HFC-43-10mee andhexamethyldisiloxane
Hexamethyldisiloxane (HMDSO) is an organosilicon compound with the formula O i(CH3)3sub>2. This volatile colourless liquid is used as a solvent and as a reagent in organic synthesis. It is prepared by the hydrolysis of trimethylsilyl chloride. ...
can dissolve silicones and are highly compatible with polycarbonates and polyurethane. They can be used to remove silicone lubricant from medical devices.
* Zeotropic mixtures of HFC-43-10mee and isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
can remove ions and water from materials without porous surfaces. This zeotropic mixture helps with absorption drying.
* Zeotropic mixtures of HFC-43-10mee, fluorosurfactant
Per- and polyfluoroalkyl substances (PFASs) are synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. An early definition, from 2011, required that they contain at least one perfluoroalkyl mo ...
, and antistatic
An antistatic agent is a compound used for treatment of materials or their surfaces in order to reduce or eliminate buildup of static electricity. Static charge may be generated by the triboelectric effect or by a non-contact process using a high ...
additives are energy-effiicient and environmentally safe drying fluids that provide spot-free drying.
See also
*List of Refrigerants
This is a list of refrigerants, sorted by their ASHRAE-designated numbers, commonly known as R numbers. Many modern refrigerants are man-made halogenated gases, especially fluorinated gases and chlorinated gases, that are frequently referred to a ...
* Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This ...
References
{{reflist Chemical engineering thermodynamics