Vinyl Iodide Functional Group on:
[Wikipedia]
[Google]
[Amazon]
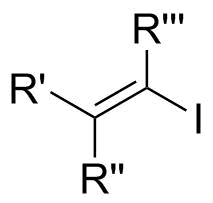 In organic chemistry, a vinyl iodide (also known as an iodoalkene) functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross- coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and
In organic chemistry, a vinyl iodide (also known as an iodoalkene) functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross- coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and
 In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the bond dissociation energies of C-I is 57.6kcal/mol, while fluoride, chloride and bromide are 115, 83.7, 72.1 kcal/mol respectively. As a result of having weaker bond, vinyl iodide does not polymerize as easily as its vinyl halide counterparts, but rather decompose and release iodide.
It is generally believed that vinyl iodide cannot survive common reduction conditions, which reduces the vinyl iodide to an
In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the bond dissociation energies of C-I is 57.6kcal/mol, while fluoride, chloride and bromide are 115, 83.7, 72.1 kcal/mol respectively. As a result of having weaker bond, vinyl iodide does not polymerize as easily as its vinyl halide counterparts, but rather decompose and release iodide.
It is generally believed that vinyl iodide cannot survive common reduction conditions, which reduces the vinyl iodide to an 
 Besides using vinyl iodides as useful substrates in transition metal cross- coupling reaction, they can also undergo
Besides using vinyl iodides as useful substrates in transition metal cross- coupling reaction, they can also undergo
 Vinyl iodides are synthesized by methods such as
Vinyl iodides are synthesized by methods such as
 Another method doesn't involve
Another method doesn't involve 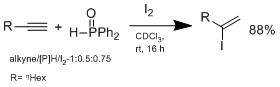 The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.
The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.

 Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
 In organic chemistry, a vinyl iodide (also known as an iodoalkene) functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross- coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and
In organic chemistry, a vinyl iodide (also known as an iodoalkene) functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross- coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and Suzuki coupling
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, a ...
. Synthesis of well-defined geometry or complexity vinyl iodide is important in stereoselective synthesis of natural products and drugs
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalat ...
.
Properties
Vinyl iodides are generally stable under nucleophilic conditions. In SN2 reactions, back-attack is difficult because of steric clash of R groups on carbon adjacent toelectrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
center (see figure 1a).Klein, David. Organic Chemistry. John Wiley & Sons, Jun 15, 2011. Google book. Thurs. 28 Nov. 2013. https://books.google.com/books?id=SsX9pbarkQkC&source=gbs_navlinks_s In addition, the lone pair on iodide donates into the ╥* of the alkene, which reduces electrophilic character on the carbon as a result of decreased positive charge. Also, this stereoelectronic effect strengthens the C-I bond, thus making removal of the iodide difficult (see figure 1b). In SN1 case, dissociation is difficult because of the strengthened C-I bond and loss of the iodide will generate an unstable carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...
(see figure 1c)
 In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the bond dissociation energies of C-I is 57.6kcal/mol, while fluoride, chloride and bromide are 115, 83.7, 72.1 kcal/mol respectively. As a result of having weaker bond, vinyl iodide does not polymerize as easily as its vinyl halide counterparts, but rather decompose and release iodide.
It is generally believed that vinyl iodide cannot survive common reduction conditions, which reduces the vinyl iodide to an
In cross- coupling reactions, typically vinyl iodides react faster and under more mild conditions than vinyl chloride and vinyl bromide. The order of reactivity is based on the strength of carbon-halogen bond. C-I bond is the weakest of the halogens, the bond dissociation energies of C-I is 57.6kcal/mol, while fluoride, chloride and bromide are 115, 83.7, 72.1 kcal/mol respectively. As a result of having weaker bond, vinyl iodide does not polymerize as easily as its vinyl halide counterparts, but rather decompose and release iodide.
It is generally believed that vinyl iodide cannot survive common reduction conditions, which reduces the vinyl iodide to an olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
or unsaturated alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which ...
. However, there is evidence in literature, in which a propargyl alcohol
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the chemical formula, formula C3H4O. It is the simplest stable Alcohol (chemistry), alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid t ...
's alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
was reduced in presence of a vinyl iodide using hydrogen over Pd/CaCO3 or Crabtree's catalyst.

Other applications
 Besides using vinyl iodides as useful substrates in transition metal cross- coupling reaction, they can also undergo
Besides using vinyl iodides as useful substrates in transition metal cross- coupling reaction, they can also undergo elimination
Elimination may refer to:
Science and medicine
* Elimination reaction, an organic reaction in which two functional groups split to form an organic product
*Bodily waste elimination, discharging feces, urine, or foreign substances from the bo ...
with a strong base to give corresponding alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
, and they can be converted to suitable vinyl Grignard reagents. Vinyl iodides are converted to Grignard reagents by magnesium-halogen exchange (see Scheme 1a).Rottlander, M.; Boymond, L.; Cahiez, G.; Knochel, P. J. Org. Chem. 1999. 64, 1080 The scope of this synthetic method is limited since it requires higher temperatures and longer reaction time, which affects functional group tolerance. However, vinyl iodide with electron withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
can enhance rate of exchange(see Scheme 1b). Also addition of lithium chloride helps enhance magnesium-halogen exchange (see Scheme 1c). It is predicted lithium chloride breaks up aggregates in organomagnesium reagents.
Methods of synthesis
 Vinyl iodides are synthesized by methods such as
Vinyl iodides are synthesized by methods such as iodination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, ...
and substitution reaction. Vinyl iodides with well-defined geometry (regiochemistry
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
and stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
) are important in synthesis since many natural products and drugs
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalat ...
that have specific structure and dimension. Example of regiochemistry
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
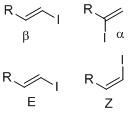
is whether the iodide is positioned in either alpha or beta position on the olefin. Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
such as E-Z notation or cis-trans alkene geometry is important since some transition metal cross- coupling reactions, such as the Suzuki coupling
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, a ...
, can retain olefin geometry. In synthesis, it is useful to introduce vinyl iodide at various positions to be set up for a coupling reaction at the next synthetic step. Below are various means and methods in introducing and synthesizing vinyl iodides.
Synthesis from alkynes
The common and simplest approach to make vinyl iodide is addition of one equivalent HI toalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
. This generally makes 2-iodo-1-alkenes or α-vinyl iodide by Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a p ...
. However, this reaction does not happen at good rates or very high stereoselectively
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
. As a result, most synthetic methods often involve a hydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
step before addition of I+ source.
α-vinyl iodides
Introducing an α-vinyl iodide from a terminal position of an alkyne is a difficult step. in addition, the vinyl metal intermediate can be mildly nucleophilic, for example vinyl aluminum, can form C-C bonds under catalytic conditions. However, Hoveyda group have demonstrated using nickel-based catalyst (Ni(dppp)Cl2), DIBAL-H with ''N''-iodosuccinimide (NIS), selectively favor α-vinyl iodide with little to no byproducts. Also they observed reverse selectivity for β with Ni(PPh3)2Cl2 in their hydroalumination reactions under same conditions with little or no byproducts. The advantage of this method is that is inexpensive (and commercially available), scalable and one-pot reaction. Another method doesn't involve
Another method doesn't involve hydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
but hydroiodation with I2/hydrophosphine binary system, which was developed by Ogawa's group.
 The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.
The hydroiodation proceeds by Markovnikov-type adduct, no reaction is observed without addition of hydrophoshine. In a plausible mechanism proposed by Ogawa's group, the hydrophosphine reacts with HI to form an intermediate complex that coordinate HI to do Markovnikov hydroiodation on the alkene. The advantage of this system is the conditions are mild, can tolerate wide range of functional groups.
β-vinyl iodides
They are generally more methods in making β-vinyl iodides versus α-vinyl iodides usinghydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
(with aluminum with DIBAL-H ( hydroalumination), with boron ( hydroboration), with HZrCp2Cl (hydrozirconation
Schwartz's reagent is the common name for the organozirconium compound with the empirical formula, formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry pr ...
)).Chong, J.; Darwish, Alla. Tetrahedron, Volume 68, Issue 2, 14 Jan 2012, pages 654-658 However, hydrometalation Hydrometalation (hydrometallation) is a type of chemical reaction in organometallic chemistry in which a chemical compound with a hydrogen to metal bond (M-H, metal hydride) adds to compounds with an unsaturated bond like an alkene (RC=CR) forming ...
with alkyne with various functional groups often react poorly with side products. The Chong groups have demonstrated using hydrostannation, using Bu3SnH with palladium catalyst with high E stereoselectivity. They observed using sterically bulky ligands gave higher regioselectivity for β-vinyl iodide. The advantage of this technique is this technique can tolerate a wide range of functional groups.
Z selective β-vinyl iodides are slightly more difficult to introduce than E-β-vinyl iodides, often requiring more than one step. Hydroalumination and hydroboration usually proceed by syn fashion, therefore selectively favors E geometry. The Oshima group have demonstrated using hydroindation with HInCl selectively favors Z geometry. They suggested that the reaction proceeds by a radical mechanism. They predict that HInCl adds to alkyne by radical addition in a Z geometry. It does not isomerized to E geometry because of low reactivity of radical InCl2 with intermediate complex (no second addition). If second addition occurs then isomerization will occur through diindium intermediate. They confirm a radical mechanism in a mechanistic study with alkyne and alkene cyclization.
Substitution
Substitution is perhaps most useful method in introducing vinyl iodide into the molecule. Halogen-exchange can be useful since vinyl iodides are more reactivity than other vinyl halides. Buchwald group demonstrates a halogen-exchange from vinyl bromide to vinyl iodide with copper catalyst under mild conditions. It is possible that this method can tolerate various functional groups since these conditions were tested aryl halides initially. The scope of this exchange forregiochemistry
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
and stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereois ...
is currently unexplored.
Halogen-exchange can also be done with zirconium derivatives that retain olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
’s geometryLiard, Annie, and Ilan Marek. "Stereoselective preparation of E vinyl zirconium derivatives from E or Z enol ethers." The Journal of organic chemistry 65.21 (2000): 7218-7220.
The Marek group have further investigated using zirconium catalyst on E or Z vinyl ether Vinyl ether may refer to:
* Any enol ether
* Divinyl ether
Divinyl ether is the organic compound with the formula O(CH=CH2)2. It is a colorless, volatile liquid that has mainly been of interest as an inhalation anesthetic. It is prepared by ...
s, which selective for E-vinyl ethers. The zirconium's oxophilic nature allows elimination alkoxy group at the β position to form intermediate vinyl zirconium complex. The E geometry selectivity is not cause by sterics but rather the reaction itself is not concerted. In a mechanistic study, they observed isomerization, which suggest E geometry product is more favored than Z geometry. The difference of results between halogen exchange and E-vinyl ether reaction is that only when there is a presence of an oxonium intermediate, is isomerization observed.
An interesting substitution reaction is vinyl boronic acid to vinyl iodide done by Brown's group. Depending on order of addition of iodide or base, vinyl borate can yield different stereoisomers of vinyl iodide (see scheme 2a). The Whiting group, however, noticed that Brown's method was not applicable to more sterically hindered boronic esters (no reaction). They proposed that the iodide source was not electropositive enough. So they decided to use ICl ICL may refer to:
Companies and organizations
* Idaho Conservation League
* Imperial College London, a UK university
* Indian Confederation of Labour
* Indian Cricket League
* Inorganic Chemistry Laboratory of the University of Oxford
* Israel Ch ...
which is more polar than I2, in which, they observed similar results (see scheme 2b).
Radical substitution of carboxylic acid to iodide is demonstrated by a modified Hunsdiecker reaction. Homolytic cleavage of O-I bond generates CO2 and vinyl radical. Vinyl radical recombines with iodide radical to form vinyl iodide.

Iododesilylation
Iododesilylation is a substitution reaction of silyl group for iodide. The advantages of iododesilylation are that it avoids toxic tin reagent and intermediate vinyl silyl are stable, nontoxic and easily handled and stored. Vinyl silyl can be made from terminal alkyne or other methods. The Kishi's group reported a mild preparation of vinyl iodide from vinyl silyl using NIS in mixture ofacetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
and chloroacetonitrile
Chloroacetonitrile is the organic compound with the formula ClCH2CN. A colorless liquid, it is derived from acetonitrile (CH3CN) by replacement of one H with Cl. In practice, it is produced by dehydration of chloroacetamide. The compound is an a ...
. They observed retention of olefin geometry in some vinyl silyl substrates while inversion in others. They reasoned that the R group's size had an effect on the geometry of the olefin. If the R group is small, the solvent acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
can participate in the reaction leading to inversion of the olefin's geometry. If the R group is big, the solvent is unable to participate, leading to retention of olefin's geometry
Zakarian's group then decided to run the reaction in HFIP, which gave high retention of olefin geometry. They reasoned that HFIP is a low nucleophilicity solvent, unlike acetonitrile. In addition, they observed accelerated reaction rate because HFIP activate NIS by hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
ing.
Unfortunately, iododesilylation under those conditions (above) can potentially yield multiple byproducts in highly functionalized molecules with oxygen functional groups. Vilarrasa and Costa's group hypothesized that radical reactions producing HI and I2 help facilitate cleavage in alcohol's protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many ...
and may add into other alkene bonds. They experimented with the use of silver additives such as silver acetate and silver carbonate in which the silver can react with the excess iodide to form silver iodide
Silver iodide is an inorganic compound with the formula Ag I. The compound is a bright yellow solid, but samples almost always contain impurities of metallic silver that give a gray coloration. The silver contamination arises because AgI is hig ...
. They achieved better conversions with these conditions.
Name reactions
Some famous vinyl iodide synthesis methods involve conversion of aldehyde orketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
to vinyl iodide. Barton's hydrazone iodination method involves addition of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
s to aldehyde or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
to form hydrazone. Then the hydrazone is converted to vinyl iodide by addition of iodide and DBU. This method has been used in natural product synthesis of Taxol by Danishefsky and Cortistatin A by Shair.
Another method is the Takai olefination
Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. It is a name reaction, referencing Kazuhiko Takai, who first reported it in 1986. In the original reaction, ...
which uses iodoform and chromium(II) chloride to make vinyl iodide from aldehyde with high stereoselectivity for E geometry. For high stereoselectivity for Z geometry, Stork-Zhao olefination proceeds by Wittig-like reaction. High yields and Z stereoselectivity occurred at low temperature and at the presence of HMPA.
 Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
Below is example of employing both Takai olefination and Stork-Zhao olefination in total synthesis of (+)-3-(E)- and (+)-3-(Z)-Pinnatifidenyne.
Elimination method
Vinyl iodides are rarely by made an elimination reaction of vicinal diiodide because it tends to decompose to alkene and iodide. The Baker group have shown using decarboxylation, elimination can occur.30. Baker, Raymond, and Jose L. Castro. "Total synthesis of (+)-macbecin I." J. Chem. Soc., Perkin Trans. 1 1 (1990): 47-65.See also
* :Functional groups *Group contribution method A group-contribution method in chemistry is a technique to estimate and predict thermodynamic and other properties from molecular structures.
Introduction
In today's chemical processes hundreds of thousands of components are used. The Chemical Ab ...
References
{{Portal bar, Chemistry, Science, Technology Alkene derivatives Chemical synthesis Organoiodides