|
Diiodide
Diiodide may refer to: * Titanium diiodide, TiI2 * Vanadium diiodide, VI2 * Chromium diiodide, CrI2 * Iron diiodide, FeI2 * Cobalt diiodide, CoI2 * Nickel diiodide, NiI2 * Germanium diiodide, GeI2 * Molybdenum diiodide, MoI2 * Palladium diiodide, PdI2 * Tin diiodide, SnI2 * Lanthanum diiodide, LaI2 * Cerium diiodide, CeI2 * Praseodymium diiodide, PrI2 * Neodymium diiodide, NdI2 * Samarium diiodide, SmI2 * Europium diiodide, EuI2 * Gadolinium diiodide, GdI2 * Dysprosium diiodide, DyI2 * Thulium diiodide, TmI2 * Ytterbium diiodide, YbI2 * Tungsten diiodide, WI2 * Osmium diiodide, OsI2 * Mercury diiodide, HgI2 * Lead diiodide, PbI2 * Thorium diiodide, ThI2 * Americium diiodide, AmI2 * Californium diiodide, CfI2 {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium Diiodide
Cerium diiodide is an iodide of cerium, with the chemical formula of CeI2. Preparation Cerium diiodide can be obtained from the reduction of cerium(III) iodide with metallic cerium under vacuum at 800 °C to 900 °C. : It can also be formed from the reaction of cerium and ammonium iodide in liquid ammonia at −78 °C. The reaction forms an ammonia complex of cerium diiodide, which decomposes to cerium diiodide under vacuum at 200 °C. : It was first created by John D. Corbett in 1961. Properties Cerium diiodide is an opaque dark solid with a metal-like appearance and properties. There is no cerium(II) in cerium diiodide, and its real structure is Ce3+(I−)2e−. It is easily hydrolyzed to form the corresponding iodide oxide. Like lanthanum diiodide and praseodymium diiodide, the cerium diiodide forms in the MoSi2-type structure, with space group In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neodymium Diiodide
Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound. Neodymium(II) iodide is a violet solid. The compound is not stoichiometric. It melts at 562°C. Preparation Neodymium(II) iodide can be made by heating molten neodymium(III) iodide with neodymium metal at 800 and 580°C for 12 hours. It can also be obtained by reducing neodymium(III) iodide with neodymium in a vacuum at 800 to 900°C: :Nd + 2NdI3 → 3NdI2 The reaction of neodymium with mercury(II) iodide is also possible because neodymium is more reactive than mercury: :Nd + HgI2 → NdI2 + Hg Direct preparation from iodine and neodymium is also possible: :Nd + I2 → NdI2 The compound was first synthesized by John D. Corbett in 1961.Angelika Jungmann, R. Claessen, R. Zimmermann, G. e. Meng, P. Steiner, S. Hüfner, S. Tratzky, K. Stöwe, H. P. Beck: ''Photoemission of LaI2 and CeI2.'' In: ''Zeitschrift für Physik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium Diiodide
Thorium diiodide is an iodide of thorium, with the chemical formula of ThI2. It is an electride with the ionic formula Th4+(I−)2e−2. It is air-sensitive. Preparation Thorium diiodide cn be prepared by heating thorium tetraiodide in stoichiometric amounts of thorium: : It can also be prepared by directly reacting thorium and iodine: : Thorium diiodide can also be prepared from the decomposition of thorium triiodide at temperatures above 550 °C: : Properties Like the diiodides of cerium, praseodymium and gadolinium Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen o ..., it has a metallic gold lustre and high electrical conductivity. References {{Actinide halides Iodides Thorium compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Praseodymium Diiodide
Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound. Preparation Praseodymium diiodide can be obtained by reacting praseodymium(III) iodide with metallic praseodymium at 800 °C to 900 °C in an inert atmosphere: :Pr + 2 PrI3 → 3 PrI2 It can also be obtained by reacting praseodymium with mercury(II) iodide where praseodymium displaces mercury: :Pr + HgI2 → PrI2 + Hg Praseodymium diiodide was first obtained by John D. Corbett in 1961. Properties Praseodymium diiodide is an opaque, bronze-coloured solid with a metallic lustre that is soluble in water. The lustre and very high conductivity can be explained by the formulation , with one electron per metal centre delocalised in a conduction band. The compound is extremely hygroscopic, and can only be stored and handled under carefull ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum Diiodide
Lanthanum diiodide is an iodide of lanthanum, with the chemical formula of LaI2. It is an electride, actually having a chemical formula of La3+ I−)2e− Preparation Lanthanum diiodide can be obtained from the reduction of lanthanum(III) iodide with lanthanum metal under a vacuum at 800 to 900 °C: : It can also be obtained by reacting lanthanum and mercury(II) iodide: : It was first created by John D. Corbett in 1961. Properties Lanthanum diiodide is a blue-black solid with metallic lustre, which is easily hydrolyzed Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis ... into the iodide oxide. It has a MoSi2-type structure, with the space group ''I''4/''mmm'' (No. 139). References {{Lanthanide halides Lanthanum compounds Iodides Electrides Substances discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinium Diiodide
Gadolinium diiodide is an inorganic compound, with the chemical formula of GdI2. It is an electride, with the ionic formula of Gd3+(I−)2e−, and therefore not a true gadolinium(II) compound. It is ferromagnetic at 276 K with a saturation magnetization of 7.3 B; it exhibits a large negative magnetoresistance Magnetoresistance is the tendency of a material (often ferromagnetic) to change the value of its electrical resistance in an externally-applied magnetic field. There are a variety of effects that can be called magnetoresistance. Some occur in bulk ... (~70%) at 7 T near room temperature. It can be obtained by reacting gadolinium and gadolinium(III) iodide at a high temperature: : Gd + 2 GdI3 → 3 GdI2 It can react with hydrogen at high temperature (800 °C) to obtain gadolinium hydrogen iodide (GdI2H0.97). References {{Lanthanide halides Gadolinium compounds Iodides Electrides Ferromagnetic materials Lanthanide halides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Diiodide
Lead(II) iodide or lead iodide is a chemical compound with the formula . At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide. The compound currently has a few specialized applications, such as the manufacture of solar cells and X-ray and gamma-ray detectors. Its preparation is an entertaining and popular demonstration in chemistry education, to teach topics such as precipitation reactions and stoichiometry. It is decomposed by light at temperatures above , and this effect has been used in a patented photographic process. Lead iodide was formerly employed as a yellow pigment in some paints, with the name iodide yellow. However, that use has been largely discontinued due to its toxicity and poor stability. Preparation is commonly synthesized via a precipitation reaction between potassium iodide and lead(II) nitrate ()2 in water solution: :Pb(NO3)2 + 2 KI → PbI2 + 2 KNO3 Wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Diiodide
Mercury(II) iodide is a chemical compound with the molecular formula Hg I2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm). Production Mercury(II) iodide is produced by adding an aqueous solution of to an aqueous solution of mercury(II) chloride with stirring; the precipitate is filtered off, washed and dried at 70 °C. : HgCl2 + 2 KI → HgI2 + 2 KClProperties Mercury(II) iodide displays |
Tungsten Diiodide
Tungsten(II) iodide is an iodide of tungsten, with the chemical formula 6I84, or abbreviated as WI2. Preparation Tungsten diiodide can obtained from the decomposition from tungsten(III) iodide: : It can also be formed by the displacement reaction of tungsten(II) chloride and iodine: : It can also be formed by the direct reaction of tungsten and iodine, which is a reversible reaction. This reaction can be used in halogen lamps. : Tungsten(II) iodide can also be obtained by reacting tungsten hexacarbonyl with iodine. Properties Tungsten(II) iodide is a dark brown-colored solid that is stable in air and moisture. Its structure is the same as tungsten(II) chloride, crystallising orthorhombic crystal system, with space group Bbem (No. 64), and lattice parameters Lattice may refer to: Arts and design * Latticework, an ornamental criss-crossed framework, an arrangement of crossing laths or other thin strips of material * Lattice (music), an organized grid model of pitch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thulium Diiodide
Thulium(II) iodide is an inorganic compound with the chemical formula TmI2. Preparation and properties Thulium(II) iodide can be obtained by reacting thulium(III) iodide and alkali metals in a tantalum container at 700~800°C. TmI2 can be obtained by using lithium and sodium as reducing agents, while potassium, rubidium and caesium can generate the perovskite structure MTmI3. TmI2 can also react directly with CsI (340 °C) to generate CsTmI3.罗时敏,王世华.CsTmI3生成机理及其相转变 物理化学学报,1990(01):93-96. Thulium(II) iodide can also be prepared by reacting thulium and mercury(I) iodide Mercury(I) iodide is a chemical compound of mercury and iodine. The chemical formula is Hg2I2. It is photosensitive and decomposes easily to mercury and HgI2. Synthesis Mercury(I) iodide can be prepared by directly reacting mercury and iodine. .... The reaction process is as follows: : : : References {{Iodides Thulium compounds Iodides Lanthanide ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
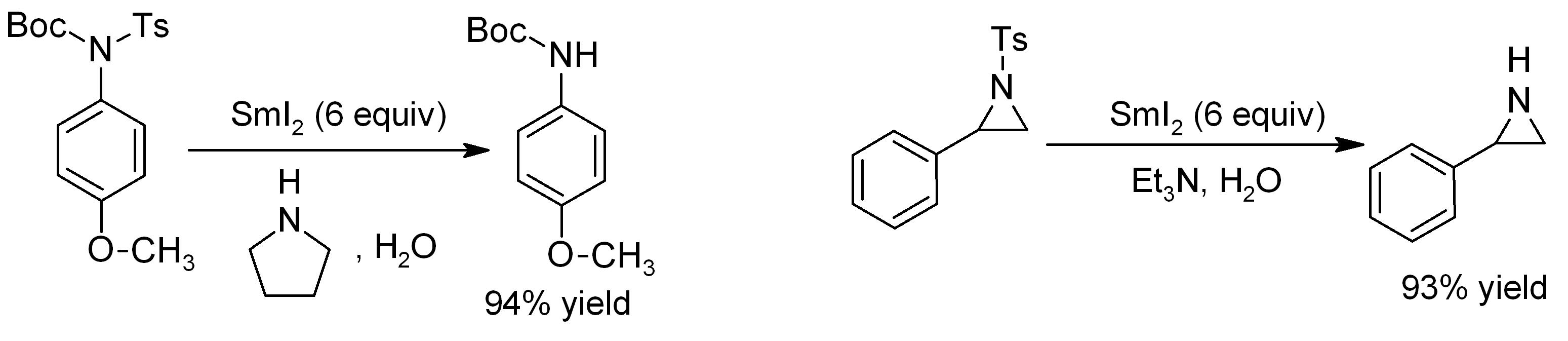
Samarium Diiodide
Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and solutions are green as well. It is a strong one-electron reducing agent that is used in organic synthesis. Structure In samarium(II) iodide, the metal centers are seven-coordinate with a face-capped octahedral geometry. In its ether adducts, samarium remains heptacoordinate with five ether and two terminal iodide ligands. Preparation Samarium iodide is easily prepared in nearly quantitative yields from samarium metal and either diiodomethane or 1,2-diiodoethane. When prepared in this way, its solutions is most often used without purification of the inorganic reagent. Solid, solvent-free SmI2 forms by high temperature decomposition of samarium(III) iodide (SmI3).G. Jantsch, N. Skalla: "Zur Kenntnis der Halogenide der seltenen Erden. IV. – Über Samarium(II)jodid und den thermischen Abbau des Samariu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Americium Diiodide
Americium(II) iodide is the inorganic compound with the formula AmI2. It is a black solid which crystallizes in the same motif as strontium bromide Strontium bromide is a chemical compound with a formula SrBr2. At room temperature it is a white, odourless, crystalline powder. Strontium bromide imparts a bright red colour in a flame test, showing the presence of strontium ions. It is used in f .... References Americium compounds Iodides Actinide halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |